Water absorption
Encyclopedia

Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
through a medium containing water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
molecules, portions of the electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
are absorbed
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
by water molecules. This water absorption occurs preferentially at certain characteristic wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
s while the balance of the spectrum is transmitted
Transmittance
In optics and spectroscopy, transmittance is the fraction of incident light at a specified wavelength that passes through a sample. A related term is absorptance, or absorption factor, which is the fraction of radiation absorbed by a sample at a specified wavelength...
with minimal effects.
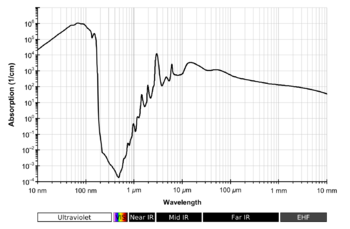
Strong absorbance by water vapor
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
occurs at wavelengths around 2900, 1950 and 1450 nanometers (nm),
with weaker absorption around 1200 and 970 nm,
and three additional sets of water-vapor absorption lines near 930, 820, and 730 nm,
all in the infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
spectrum. Water has a complex absorption spectrum — the 2007 HITRAN
HITRAN
HITRAN - HITRAN is a compilation of spectroscopic parameters that a variety of computer codes use to predict and simulate the transmission and emission of light in the atmosphere. The original version was compiled by the Air Force Cambridge Research Laboratories...
spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
database update lists more than 64,000 spectral line
Spectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies.- Types of line spectra :...
s corresponding to significant transitions of water vapor ranging from the microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
region to the visible spectrum
Visible spectrum
The visible spectrum is the portion of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelengths is called visible light or simply light. A typical human eye will respond to wavelengths from about 390 to 750 nm. In terms of...
.
The spectral absorption features of liquid water are shifted to longer wavelengths with respect to the vapor features by approximately 60 nm
Nanometre
A nanometre is a unit of length in the metric system, equal to one billionth of a metre. The name combines the SI prefix nano- with the parent unit name metre .The nanometre is often used to express dimensions on the atomic scale: the diameter...
. In hexagonal ice, the features are shifted even further into the red and infrared. In liquid water and ice the infrared and Raman spectra
Raman spectroscopy
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
are far more complex than in the vapor.
Atmospheric effects
Water vapor is a greenhouse gasGreenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
in the Earth's atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
, responsible for 70% of the known absorption of incoming sunlight
Sunlight
Sunlight, in the broad sense, is the total frequency spectrum of electromagnetic radiation given off by the Sun. On Earth, sunlight is filtered through the Earth's atmosphere, and solar radiation is obvious as daylight when the Sun is above the horizon.When the direct solar radiation is not blocked...
, particularly in the infrared region, and about 60% of the atmospheric absorption of thermal radiation
Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation....
by the Earth known as the greenhouse effect
Greenhouse effect
The greenhouse effect is a process by which thermal radiation from a planetary surface is absorbed by atmospheric greenhouse gases, and is re-radiated in all directions. Since part of this re-radiation is back towards the surface, energy is transferred to the surface and the lower atmosphere...
. It is also an important factor in multispectral imaging and hyperspectral imaging
Hyperspectral imaging
Hyperspectral imaging collects and processes information from across the electromagnetic spectrum. Much as the human eye sees visible light in three bands , spectral imaging divides the spectrum into many more bands...
used in remote sensing
Remote sensing
Remote sensing is the acquisition of information about an object or phenomenon, without making physical contact with the object. In modern usage, the term generally refers to the use of aerial sensor technologies to detect and classify objects on Earth by means of propagated signals Remote sensing...
because water vapor absorbs radiation differently in different spectral bands. Its effects are also an important consideration in infrared astronomy
Infrared astronomy
Infrared astronomy is the branch of astronomy and astrophysics that studies astronomical objects visible in infrared radiation. The wavelength of infrared light ranges from 0.75 to 300 micrometers...
and radio astronomy
Radio astronomy
Radio astronomy is a subfield of astronomy that studies celestial objects at radio frequencies. The initial detection of radio waves from an astronomical object was made in the 1930s, when Karl Jansky observed radiation coming from the Milky Way. Subsequent observations have identified a number of...
in the microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
or millimeter wave bands. The South Pole Telescope
South Pole Telescope
The South Pole Telescope is a 10 metre diameter telescope located at the Amundsen-Scott South Pole Station, Antarctica. It is a microwave/millimetre-wave telescope that observes in a frequency range between 70 and 300 GHz...
was constructed in Antarctica in part because the elevation and low temperatures there mean there is very little water vapor in the atmosphere.
Similarly, carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
absorption bands occur around 1400, 1600 and 2000 nm, but its presence in the Earth's atmosphere accounts for just 26% of the greenhouse effect. Carbon dioxide gas absorbs energy in some small segments of the thermal infrared spectrum that water vapor misses. This extra absorption within the atmosphere causes the air to warm just a bit more and the warmer the atmosphere the greater its capacity to hold more water vapor. This extra water vapor absorption further enhances the Earth’s greenhouse effect.
In the atmospheric window
Atmospheric window
The infrared atmospheric window is the overall dynamic property of the earth's atmosphere, taken as a whole at each place and occasion of interest, that lets some infrared radiation from the cloud tops and land-sea surface pass directly to space without intermediate absorption and re-emission, and...
between approximately 8000 and 14000 nm, in the far-infrared spectrum, carbon dioxide and water absorption is weak. This window allows most of the thermal radiation in this band to be radiated out to space, keeping the Earth's atmosphere from going into thermal runaway
Thermal runaway
Thermal runaway refers to a situation where an increase in temperature changes the conditions in a way that causes a further increase in temperature, often leading to a destructive result...
. This band is also used for remote sensing of the Earth from space, for example with thermal Infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
imaging.
Physical explanation
The absorption bands of water vapor are related to molecular vibrationMolecular vibration
A molecular vibration occurs when atoms in a molecule are in periodic motion while the molecule as a whole has constant translational and rotational motion...
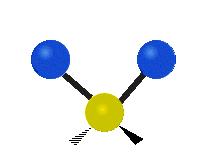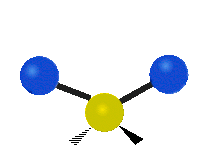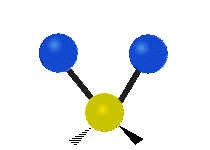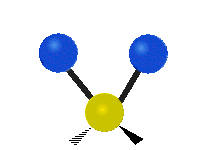
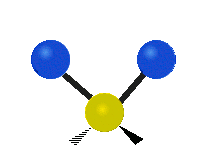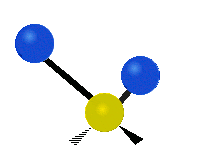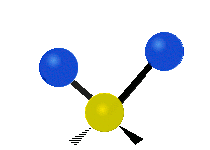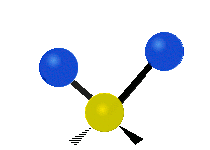
s involving various combinations of the water molecule's three fundamental vibrational modes:
- V1: symmetric stretch
- V2: bending
- V3: asymmetric stretch
The absorption feature centered near 970 nm is attributed to a 2V1 + V3 combination, the one near 1200 nm to a V1 + V2 + V3 combination, the one near 1450 nm to a V1 + V3 combination, and the
one near 1950 nm to a V2 + V3 combination.
In liquid water, rotations tend to be hindered by hydrogen bonds, leading to librations
Libration (molecule)
Libration is a type of reciprocating motion in which an object with a nearly fixed orientation repeatedly rotates slightly back and forth...
, or rocking motions. Stretching vibrations are shifted to a lower frequency while the bending frequency increases due to hydrogen bonding.
Three fundamental vibrations of the water molecule
|
|---|
See also
- Hydroxyl ion absorptionHydroxyl ion absorptionHydroxyl ion absorption is the absorption in optical fibers of electromagnetic waves, including the near-infrared, due to the presence of trapped hydroxyl ions remaining from water as a contaminant....
in optical fiberOptical fiberAn optical fiber is a flexible, transparent fiber made of a pure glass not much wider than a human hair. It functions as a waveguide, or "light pipe", to transmit light between the two ends of the fiber. The field of applied science and engineering concerned with the design and application of... - Color of waterColor of waterThe color of water is a subject of both scientific study and popular misconception. While relatively small quantities of water are observed by humans to be colorless, pure water has a slight blue color that becomes a deeper blue as the thickness of the observed sample increases...
- Water modelWater modelIn computational chemistry, classical water models are used for the simulation of water clusters, liquid water, and aqueous solutions with explicit solvent. These models use the approximations of molecular mechanics...