
1,3,2,4-Dithiadiphosphetane 2,4-disulfides
Encyclopedia
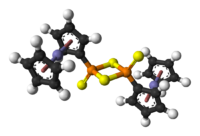
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
which contain a P2S2 ring, many of these compounds are able to act as sources of the dithiophosphine ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
s. The most well known example of this class of compound is Lawesson's reagent
Lawesson's reagent
Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with...
.
Other examples of this class of compound have been made; many inorganic chemists are now using Fc2P2S4 (Fc = ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
) as a starting material in reactions investigating the general chemistry of the 1,3,2,4-dithiadiphosphetane 2,4-disulfides, one reaction for this is that the Fc2P2S4 compound and all its derivatives are red which make column chromatography of the products more easy. Also the ferrocenyl groups provide an electrochemical handle which provide another means of investigating the properties of the products.
Examples
While several different routes to the 1,3,2,4-dithiadiphosphetane 2,4-disulfides exist the most commonly used is the electrophilic aromatic substitution reaction of an arene with P4S10. An alternative reaction is the reaction of a thiolThiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
with P4S10 to form a substance like the Davy reagent. The Davy reagent is identical to Lawesson's reagent except in place of the para-methoxyphenyl groups it has aryl sulfide groups. While the Davy reagent is more soluble than the Lawesson's reagent it is likely that the very vile
Vile
- People :* Tommy Vile , Welsh international rugby union player* William Vile , English cabinetmaker* Katie Biondo , Un-talented Dancer- Mythology :* Vile, Vilae: Etruscan mythological name for Greek Iolaus, nephew of Hercules...
nature of the thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
starting material is likely to make the synthesis of this compound not worth the trouble. In both the patent
Patent
A patent is a form of intellectual property. It consists of a set of exclusive rights granted by a sovereign state to an inventor or their assignee for a limited period of time in exchange for the public disclosure of an invention....
and academic chemical literature are examples of 1,3,2,4-dithiadiphosphetane 2,4-disulfides with higher solubilities. These highly soluble versions of Lawesson's reagent are created by the reaction of P4S10 with aryl ethers which are different from anisole
Anisole
Anisole, or methoxybenzene, is the organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances...
. For instance butoxybenzene and 2-tert-butylanisole have both been reacted to form more soluble thionation reagents of the 1,3,2,4-dithiadiphosphetane 2,4-disulfide class.
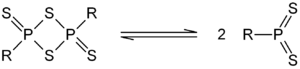
Naphthalen-1,8-diyl 1,3,2,4-dithiadiphosphetane 2,4-disulfide
A compound related to Lawesson's reagent named NpP2S4 has been formed by the reaction of 1-bromonaphthalene with P4S10, this is a 1,3,2,4-dithiadiphosphetane 2,4-disulfide which has a naphth-1,8-diyl group holding the two phosphorus atoms together...
s; these are intellectually interesting because the two dithiophosphine ylides are fixed together in space by the rigid naphthalene unit. The reactivity of these compounds is very different from that of 1,3,2,4-dithiadiphosphetane 2,4-disulfides.
Reactions
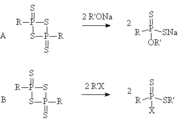
The dithiophosphine ylides are normally attacked at the phosphorus atom by a nucleophileNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
, for instance the reaction of an alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
, phenolate, alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
or phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
with a 1,3,2,4-dithiadiphosphetane 2,4-disulfide can form a new compound with a phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
-oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
bond. Such a reaction has been used in the formation of metal binding agents and in the synthesis of insecticide
Insecticide
An insecticide is a pesticide used against insects. They include ovicides and larvicides used against the eggs and larvae of insects respectively. Insecticides are used in agriculture, medicine, industry and the household. The use of insecticides is believed to be one of the major factors behind...
s.
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
with 1,3,2,4-dithiadiphosphetane 2,4-disulfides is less common, but the reaction of an alkyl halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
with a 1,3,2,4-dithiadiphosphetane 2,4-disulfide forms a new compound with a sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
-carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
and a phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
-halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
bond. Such a compound could act as an acetylcholinesterase
Acetylcholinesterase
"Acetylcholinesterase, also known as AChE or acetylcholine acetylhydrolase, is an enzyme that degrades the neurotransmitter acetylcholine, producing choline and an acetate group. It is mainly found at neuromuscular junctions and cholinergic nervous system, where its activity serves to terminate...
inhibitor
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
in insects, but in order to make a better insecticide
Insecticide
An insecticide is a pesticide used against insects. They include ovicides and larvicides used against the eggs and larvae of insects respectively. Insecticides are used in agriculture, medicine, industry and the household. The use of insecticides is believed to be one of the major factors behind...
it would be best to convert the halide to another leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
which would form a less water sensitive product. For instance the reaction of para-nitrophenolate would form a compound similar to parathion
Parathion
Parathion, also called parathion-ethyl or diethyl parathion, is an organophosphate compound. It is a potent insecticide and acaricide. It was originally developed by IG Farben in the 1940s. It is highly toxic to non-target organisms, including humans. Its use is banned or restricted in many...
.
Lawesson's reagent has been used as a starting material for a herbicide
Herbicide
Herbicides, also commonly known as weedkillers, are pesticides used to kill unwanted plants. Selective herbicides kill specific targets while leaving the desired crop relatively unharmed. Some of these act by interfering with the growth of the weed and are often synthetic "imitations" of plant...
by reaction with a 1-alkoxy-2,3-dihydroxy propane. This formed a compound which could be used to kill plants. This reaction of a 1,2-diol with lawesson's reagent results in a symmetric breaking of the P2S2 ring, both halves of the lawesson's reagent end up being converted to the same product.
A different type of ring breaking reaction can occur when LR is reacted with a metal compounds such as a platinum dichloride bis-phosphine complex, in this case one molecule of MeOC6H4P(S)Cl2 is formed as a side product to the platinum complex ([Pt(S2P(S)C6H4OMe)(PR3)2]).
Lawesson's reagent can be used as a dehydrating reagent, for example it has been used to convert a β-aminoamide into an imidazoline.
Another useful reaction of LR is the conversion of a 1,4-diketone into a thiophene ring, this reaction can be done with P4S10 but a much higher temperature would be required to make it work with P4S10.
It was claimed in a German patent that the reaction of 1,3,2,4-dithiadiphosphetane 2,4-disulfides with dialkyl cyanamides formed plant protection agents which contained six-membered (P-N=C-N=C-S-) rings. It has been proven in recent times by the reaction of diferrocenyl 1,3,2,4-dithiadiphosphetane 2,4-disulfide (and Lawesson's reagent) with dimethyl cyanamide
Cyanamide
Cyanamide is an organic compound with the formula CN2H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol deterrent drug in Canada, Europe and Japan. The molecule features a nitrile group attached to an...
that in fact a mixture of several different phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
containing compounds is formed. Depending on the concentration of the dimethyl cyanamide in the reaction mixture either a different six membered ring compound (P-N=C-S-C=N-) or a nonheterocylic compound (FcP(S)(NR2)(NCS)) is formed as the major product, the other compound is formed as a minor product.
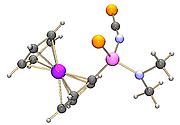
Nerve
A peripheral nerve, or simply nerve, is an enclosed, cable-like bundle of peripheral axons . A nerve provides a common pathway for the electrochemical nerve impulses that are transmitted along each of the axons. Nerves are found only in the peripheral nervous system...
poisons in insects. These compounds bearing terminal sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
atoms on the phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
atom are much less toxic than the compounds (such as sarin
Sarin
Sarin, or GB, is an organophosphorus compound with the formula [2CHO]CH3PF. It is a colorless, odorless liquid, which is used as a chemical weapon. It has been classified as a weapon of mass destruction in UN Resolution 687...
, VX
VX (nerve agent)
VX, IUPAC name O-ethyl S-[2-ethyl] methylphosphonothioate, is an extremely toxic substance whose only application is in chemical warfare as a nerve agent. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations in UN Resolution 687...
and tetraethyl pyrophosphate
Tetraethyl pyrophosphate
Tetraethyl pyrophosphate, abbreviated TEPP, is an organophosphate compound. It is used as a pesticide.This compound is a clear, colorless liquid. It is soluble in water, but hydrolyzes rapidly. It was first synthesized by Philippe de Clermont...
) which have an oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
in place of this terminal sulfur. This is because the P=S compound is not active as an acetylcholinesterase
Acetylcholinesterase
"Acetylcholinesterase, also known as AChE or acetylcholine acetylhydrolase, is an enzyme that degrades the neurotransmitter acetylcholine, producing choline and an acetate group. It is mainly found at neuromuscular junctions and cholinergic nervous system, where its activity serves to terminate...
inhibitor
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
in either mammals or insects, in mammals the animals metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
tends to remove lipophilic
Lipophilic
Lipophilicity, , refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. These non-polar solvents are themselves lipophilic — the axiom that like dissolves like generally holds true...
side groups from the phosphorus atom while an insect tends to oxidise the compound so removing the terminal sulfur and replacing it with a terminal oxygen which causes the compound to be more able to act as an acetylcholinesterase inhibitor.
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
the small rings containing more heavy elements such as sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
are more stable with regards to ring opening. Hence, the rings such as PSC2 are much more stable than things like epoxides.
A selenium version of this ring type has been made, one notable example has been named Woollins' reagent and is Ph2P2Se4, this is made by the reaction of (PhP)5 with selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
metal. The solubility of this compound is very low but the group of Prof J. Derek Woollins have published some reactions of this compound. For instance the reaction of Woollins' reagent (WR) with a dialkyl cyanamide has been found to form a bicyclic PC2N2Se3 system.

