
Carbamoyl phosphate synthetase
Encyclopedia
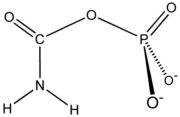
of carbamoyl phosphate
Carbamoyl phosphate
Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals it is an intermediary metabolite participating in the nitrogen disposal through in the urea cycle and the synthesis of pyrimidines....
from glutamine
Glutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
or ammonia and bicarbonate. This enzyme catalyzes the reaction of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
and bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
to produce carbonyl phosphate and ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
. Carbonyl phosphate reacts with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
to give carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
. In turn, carbamate reacts with a second ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
to give carbamoyl phosphate
Carbamoyl phosphate
Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals it is an intermediary metabolite participating in the nitrogen disposal through in the urea cycle and the synthesis of pyrimidines....
plus ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
.
It represents the first committed step in pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
and arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
biosynthesis
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
in prokaryotes and eukaryotes, and in the urea cycle
Urea cycle
The urea cycle is a cycle of biochemical reactions occurring in many animals that produces urea from ammonia . This cycle was the first metabolic cycle discovered , five years before the discovery of the TCA cycle...
in most terrestrial vertebrates. Most prokaryotes
Prokaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
carry one form of CPSase that participates in both arginine and pyrimidine biosynthesis, however certain bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
can have separate forms.
There are three different forms that serve very different functions:
- Carbamoyl phosphate synthetase ICarbamoyl phosphate synthetase ICarbamoyl Phosphate Synthetase I is a ligase enzyme located in the mitochondria involved in the production of urea. Carbamoyl Phosphate Synthetase I transfers an ammonia from glutamine to a molecule of bicarbonate that has been phosphorylated by a molecule of ATP. The resulting carbamate is...
(mitochondria, urea cycleUrea cycleThe urea cycle is a cycle of biochemical reactions occurring in many animals that produces urea from ammonia . This cycle was the first metabolic cycle discovered , five years before the discovery of the TCA cycle...
) - Carbamoyl phosphate synthetase II (cytosol, pyrimidine metabolismPyrimidine metabolismPyrimidine biosynthesis occurs both in the body and through organic synthesis.-De novo biosynthesis of pyrimidine :Unlike purines, pyrimidines are assembled before being attached to 5-phosphoribosyl-1-pyrophosphate ....
). - Carbamoyl phosphate synthetase III (found in fish).
Mechanism
Carbamoyl phosphate synthase has three main steps in its mechanism and is, in essence, irreversible.- Bicarbonate ion is phosphorylated with ATP to create carboxylphosphate.
- The carboxylphosphate then reacts with ammonia to form carbamic acid, releasing inorganic phosphate.
- A second molecule of ATP then phosphorylates carbamic acid, creating carbamoyl phosphate.
The activity of the enzyme is known to be inhibited by both Tris
Tris
Tris is an abbreviation of the organic compound known as trisaminomethane, with the formula 3CNH2. Tris is extensively used in biochemistry and molecular biology. In biochemistry, tris is widely used as a component of buffer solutions, such as in TAE and TBE buffer, especially for solutions of...
and HEPES
HEPES
HEPES is a zwitterionic organic chemical buffering agent; one of the twelve Good's buffers...
buffers.
Structure
Carbamoyl phosphatePhosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
synthase
Synthase
In biochemistry, a synthase is an enzyme that catalyses a synthesis process.Following the EC number classification, they belong to the group of ligases, with lyases catalysing the reverse reaction....
(CPSase) is a heterodimeric
Protein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
composed of a small and a large subunit (with the exception of CPSase III, which is composed of a single polypeptide that may have arisen from gene fusion of the glutaminase and synthetase domains
Protein domain
A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
). CPSase has three active sites
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
, one in the small subunit and two in the large subunit. The small subunit contains the glutamine
Glutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
binding site
Binding site
In biochemistry, a binding site is a region on a protein, DNA, or RNA to which specific other molecules and ions—in this context collectively called ligands—form a chemical bond...
and catalyses
Biocatalysis
Biocatalysis is the use of natural catalysts, such as protein enzymes, to perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task....
the hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of glutamine to glutamate and ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, which in turn
Turn (biochemistry)
A turn is an element of secondary structure in proteins where the polypeptide chain reverses its overall direction.- Definition :According to the most common definition, a turn is a structural motif where the Cα atoms of two residues separated by few peptide bonds are in close approach A turn is...
used by the large chain
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
to synthesize carbamoyl phosphate. The small subunit has a 3-layer beta/beta/alpha structure, and is thought to be mobile in most proteins
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
that carry it. The C-terminal domain of the small subunit of CPSase has glutamine amidotransferase activity. The large subunit has two homologous
Homology (biology)
Homology forms the basis of organization for comparative biology. In 1843, Richard Owen defined homology as "the same organ in different animals under every variety of form and function". Organs as different as a bat's wing, a seal's flipper, a cat's paw and a human hand have a common underlying...
carboxy phosphate domains, both of which have ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
-binding sites; however, the N-terminal carboxy phosphate domain
Protein domain
A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
catalyses the phosphorylation
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
of biocarbonate, while the C-terminal domain catalyses the phosphorylation of the carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
intermediate
Reaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
. The carboxy phosphate domain found duplicated in the large subunit of CPSase is also present as a single copy in the biotin
Biotin
Biotin, also known as Vitamin H or Coenzyme R, is a water-soluble B-complex vitamin discovered by Bateman in 1916. It is composed of a ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring...
-dependent enzymes acetyl-CoA carboxylase
Acetyl-CoA carboxylase
Acetyl-CoA carboxylase is a biotin-dependent enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA through its two catalytic activities, biotin carboxylase and carboxyltransferase...
(ACC), propionyl-CoA carboxylase
Propionyl-CoA carboxylase
Propionyl-CoA carboxylase catalyses the carboxylation reaction of propionyl CoA in the mitochondrial matrix. The enzyme is biotin dependent. The product of the reaction is -methylmalonyl CoA. Propionyl CoA is the end product of metabolism of odd-chain fatty acids, and is also a metabolite of most...
(PCCase), pyruvate carboxylase
Pyruvate carboxylase
Pyruvate carboxylase is an enzyme of the ligase class that catalyzes the irreversible carboxylation of pyruvate to form oxaloacetate .It is an important anaplerotic reaction that creates oxaloacetate from pyruvate...
(PC) and urea carboxylase
Urea carboxylase
In enzymology, an urea carboxylase is an enzyme that catalyzes the chemical reactionThe 3 substrates of this enzyme are ATP, urea, and HCO3-, whereas its 3 products are ADP, phosphate, and urea-1-carboxylate ....
.
The large subunit in bacterial CPSase has four structural
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
domains: the carboxy phosphate domain 1, the oligomerisation domain, the carbamoyl phosphate domain 2 and the allosteric domain. CPSase heterodimers from Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
contain two molecular tunnels: an ammonia tunnel and a carbamate tunnel. These inter-domain tunnels connect the three distinct active sites, and function as conduits for the transport
Transport
Transport or transportation is the movement of people, cattle, animals and goods from one location to another. Modes of transport include air, rail, road, water, cable, pipeline, and space. The field can be divided into infrastructure, vehicles, and operations...
of unstable reaction intermediates (ammonia and carbamate) between successive active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
s. The catalytic mechanism of CPSase involves the diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of carbamate through the interior of the enzyme from the site of synthesis within the N-terminal domain of the large subunit to the site of phosphorylation within the C-terminal domain.

