Clathrate hydrate
Encyclopedia
Clathrate hydrates
are crystal
line water-based solid
s physically resembling ice
, in which small non-polar
molecule
s (typically gas
es) or polar
molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bond
ed water molecules. In other words, clathrate hydrates are clathrate compounds in which the host molecule is water
and the guest molecule is typically a gas or liquid. Without the support of the trapped molecules, the lattice
structure of hydrate clathrates would collapse into conventional ice crystal structure or liquid water. Most low molecular weight gases (including 2, 2, 2, CO2
, CH4
, H2S
, , , and ), as well as some higher hydrocarbon
s and freons will form hydrate
s at suitable temperatures and pressures. Clathrate hydrates are not chemical compounds as the sequestered molecules are never bonded to the lattice. The formation and decomposition of clathrate hydrates are first order phase transitions, not chemical reactions. Their detailed formation and decomposition mechanisms on a molecular level are still not well understood.
Clathrate hydrates were first documented in 1810 by Sir Humphry Davy
.
Clathrates have been found to occur naturally in large quantities. Around 6.4 trillion (i.e. 6.4x1012) tonnes of methane
is trapped in deposits of methane clathrate
on the deep ocean floor. Such deposits can be found on the Norwegian continental shelf
in the northern headwall flank of the Storegga Slide
. Clathrates can also exist as permafrost
, as at the Mallik gas hydrate field in the Mackenzie Delta
of northwestern Canadian Arctic. These natural gas hydrates are seen as a potentially vast energy resource, but an economical extraction method has so far proven elusive. Hydrocarbon
clathrates cause problems for the petroleum industry, because they can form inside gas pipelines
often resulting in plug formation. Deep sea deposition of carbon dioxide clathrate
has been proposed as a method to remove this greenhouse gas
from the atmosphere and control climate change
.
Clathrates are suspected to occur in large quantities on some outer planet
s, moons
and trans-Neptunian object
s, binding gas at fairly high temperatures.
 Gas hydrates usually form two crystallographic
Gas hydrates usually form two crystallographic
cubic structures – structure (Type) I and structure (Type) II of space groups and
and  respectively. Seldom, a third hexagonal structure of space group
respectively. Seldom, a third hexagonal structure of space group  may be observed (Type H).
may be observed (Type H).
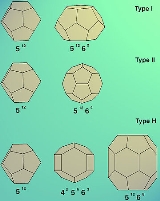
The unit cell of Type I consists of 46 water molecules, forming two types of cages – small and large. The small cages in the unit cell are two against six large ones. The small cage has the shape of a pentagonal dodecahedron (512) and the large one that of a tetradecahedron
, specifically a hexagonal truncated trapezohedron
(51262), together forming a Weaire-Phelan structure
. Typical guests forming Type I hydrates are CO2
in carbon dioxide clathrate
and CH4
in methane clathrate
.
The unit cell of Type II consists of 136 water molecules, forming also two types of cages – small and large. In this case the small cages in the unit cell are sixteen against eight large ones. The small cage has again the shape of a pentagonal dodecahedron (512) but the large one is a hexadecahedron (51264). Type II hydrates are formed by gases like O2 and N2.
The unit cell of Type H consists of 34 water molecules, forming three types of cages – two small of different type and one huge. In this case, the unit cell consists of three small cages of type 512, twelve small ones of type 435663 and one huge of type 51268. The formation of Type H requires the cooperation of two guest gases (large and small) to be stable. It is the large cavity that allows structure H hydrates to fit in large molecules (e.g. butane
, hydrocarbon
s), given the presence of other smaller help gases to fill and support the remaining cavities. Structure H hydrates were suggested to exist in the Gulf of Mexico. Thermogenically-produced supplies of heavy hydrocarbons are common there.
deficiency in comet
s, stated most of the conditions for hydrate formation in the protoplanetary nebulae
, surrounding the pre-main and main sequence
stars were fulfilled, despite the rapid grain growth to meter scale. The key was to provide enough microscopic ice particles exposed to a gaseous environment. Observations of the radiometric
continuum
of circumstellar discs
around -Tauri
-Tauri
and Herbig Ae/Be stars suggest massive dust disks consisting of millimeter-sized grains, which disappear after several million years (e.g.,). A lot of work on detecting water ices in the Universe was done on the Infrared Space Observatory
(ISO). For instance, broad emission bands
of water ice at 43 and 60 μm were found in the disk of the isolated Herbig Ae/Be star HD 100546 in Musca
. The one at 43 μm is much weaker than the one at 60 μm, which means the water ice, is located in the outer parts of the disk at temperatures below 50 K. There is also another broad ice feature between 87 and 90 μm, which is very similar to the one in NGC 6302
(the Bug or Butterfly nebula in Scorpius
). Crystalline ices were also detected in the proto-planetary disks of ε-Eridani
and the isolated Fe star HD 142527 in Lupus
. 90 % of the ice in the latter was found crystalline at temperature around 50 K. HST
demonstrated that relatively old circumstellar disks
, as the one around the 5 million year old B9.5Ve Herbig Ae/Be star HD 141569A, are dusty. Li & Lunine found water ice there. Knowing the ices usually exist at the outer parts of the proto-planetary nebulae
, Hersant et al. proposed an interpretation of the volatile enrichment, observed in the four giant planets
of the Solar System
, with respect to the Solar abundances
. They assumed the volatiles had been trapped in the form of hydrates and incorporated in the planetesimal
s flying in the protoplanets’
feeding zones.
Kieffer et al. (2006) suggest that the geyser activity in the south polar region of Saturn
's moon Enceladus
originates from clathrate hydrates, where carbon dioxide, methane, and nitrogen are released when exposed to the vacuum of space by the "Tiger Stripe
" fractures found in that area.
Carbon dioxide clathrate
is believed to play a major role in different processes on Mars. Hydrogen clathrate
is likely to form in condensation nebulae for gas giants.
gas hydrates can be found on the seafloor, in ocean sediments, in deep lake sediments (e.g. Lake Baikal
), as well as in the permafrost
regions. The amount of methane
potentially trapped in natural methane hydrate
deposits may be significant (1015 to 1017 cubic metres), which makes them of major interest as a potential energy resource. Catastrophic release of methane from the decomposition of such deposits may lead to a global climate change, because CH
4 is more efficient greenhouse gas even than CO
2 (see Atmospheric methane
). The fast decomposition of such deposits is considered a geohazard
, due to its potential to trigger landslide
s, earthquake
s and tsunami
s. However, natural gas hydrates do not contain only methane but also other hydrocarbon
gases, as well as H2S
and CO2
. Air hydrates are frequently observed in polar ice samples.
Pingo
s are common structures in permafrost regions. Similar structures are found in deep water related to methane leakages.
It is important to notice that gas hydrates can even be formed in the absence of a liquid phase. Under that situation, water is dissolved in gas or in liquid hydrocarbon phase .
. This is highly undesirable because the clathrate crystals might agglomerate and plug the flowline and cause flow assurance
failure and damage valves and instrumentation. The results can range from flow reduction to equipment damage.
and to adhere to the pipe wall and thereby plug the pipeline. Once formed, they can be decomposed by increasing the temperature and/or decreasing the pressure. Even under these conditions, the clathrate dissociation is a slow process.
Therefore, preventing hydrate formation appears to be the key to the problem. A hydrate prevention philosophy could typically be based on three levels of security, listed in order of priority:
The actual philosophy would depend on operational circumstances such as pressure, temperature, type of flow (gas, liquid, presences of water etc.)
The most common thermodynamic inhibitors are, methanol
, monoethylene glycol
(MEG) and diethylene glycol
(DEG) commonly referred to as glycol. All may be recovered and recirculated, but the economics of methanol recovery is not favourable in most cases. MEG is preferred over DEG for applications where the temperature is expected to be −10 °C or lower due to high viscosity at low temperatures. Triethylene glycol
(TEG) has too low vapour pressure to be suited as an inhibitor injected into a gas stream. More methanol is lost in the gas phase when compared to MEG or DEG.
The use of kinetic inhibitors and anti-agglomerants in actual field operations is a new and evolving technology. It requires extensive tests and optimisation to the actual system. While kinetic inhibitors work by slowing down the kinetics of the nucleation, anti-agglomerants do not stop the nucleation, they rather stop the agglomeration (sticking together) of gas hydrate crystals. These two kinds of inhibitors are also known as Low-Dosage-Hydrate-Inhibitors because they require much smaller concentrations than the conventional thermodynamic inhibitors. Kinetic inhibitors (which do not require water and hydrocarbon mixture to be effective) are usually polymers or copolymers and anti-agglomerants (requires water and hydrocarbon mixture) are polymers or zwitterionic (usually ammonium and COOH) surfactants being both attracted to hydrates and hydrocarbons.
are crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
line water-based solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
s physically resembling ice
Ice
Ice is water frozen into the solid state. Usually ice is the phase known as ice Ih, which is the most abundant of the varying solid phases on the Earth's surface. It can appear transparent or opaque bluish-white color, depending on the presence of impurities or air inclusions...
, in which small non-polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s (typically gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
es) or polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ed water molecules. In other words, clathrate hydrates are clathrate compounds in which the host molecule is water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
and the guest molecule is typically a gas or liquid. Without the support of the trapped molecules, the lattice
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
structure of hydrate clathrates would collapse into conventional ice crystal structure or liquid water. Most low molecular weight gases (including 2, 2, 2, CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, CH4
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
, H2S
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
, , , and ), as well as some higher hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s and freons will form hydrate
Hydrate
Hydrate is a term used in inorganic chemistry and organic chemistry to indicate that a substance contains water. The chemical state of the water varies widely between hydrates, some of which were so labeled before their chemical structure was understood....
s at suitable temperatures and pressures. Clathrate hydrates are not chemical compounds as the sequestered molecules are never bonded to the lattice. The formation and decomposition of clathrate hydrates are first order phase transitions, not chemical reactions. Their detailed formation and decomposition mechanisms on a molecular level are still not well understood.
Clathrate hydrates were first documented in 1810 by Sir Humphry Davy
Humphry Davy
Sir Humphry Davy, 1st Baronet FRS MRIA was a British chemist and inventor. He is probably best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine...
.
Clathrates have been found to occur naturally in large quantities. Around 6.4 trillion (i.e. 6.4x1012) tonnes of methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
is trapped in deposits of methane clathrate
Methane clathrate
Methane clathrate, also called methane hydrate, hydromethane, methane ice, "fire ice", natural gas hydrate or just gas hydrate, is a solid clathrate compound in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice...
on the deep ocean floor. Such deposits can be found on the Norwegian continental shelf
Norwegian continental shelf
The Norwegian continental shelf is the continental shelf over which Norway exercises sovereign rights as defined by the United Nations Convention on the Law of the Sea...
in the northern headwall flank of the Storegga Slide
Storegga Slide
The three Storegga Slides are considered to be amongst the largest known landslides. They occurred under water, at the edge of Norway's continental shelf , in the Norwegian Sea, 100 km north-west of the Møre coast, causing a very large tsunami in the North Atlantic Ocean...
. Clathrates can also exist as permafrost
Permafrost
In geology, permafrost, cryotic soil or permafrost soil is soil at or below the freezing point of water for two or more years. Ice is not always present, as may be in the case of nonporous bedrock, but it frequently occurs and it may be in amounts exceeding the potential hydraulic saturation of...
, as at the Mallik gas hydrate field in the Mackenzie Delta
Mackenzie River
The Mackenzie River is the largest river system in Canada. It flows through a vast, isolated region of forest and tundra entirely within the country's Northwest Territories, although its many tributaries reach into four other Canadian provinces and territories...
of northwestern Canadian Arctic. These natural gas hydrates are seen as a potentially vast energy resource, but an economical extraction method has so far proven elusive. Hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
clathrates cause problems for the petroleum industry, because they can form inside gas pipelines
Pipeline transport
Pipeline transport is the transportation of goods through a pipe. Most commonly, liquids and gases are sent, but pneumatic tubes that transport solid capsules using compressed air are also used....
often resulting in plug formation. Deep sea deposition of carbon dioxide clathrate
Carbon dioxide clathrate
Carbon dioxide hydrate is a Type I gas clathrate . However, there has been some experimental evidence for the development of a metastable Type II phase at temperature near the ice melting point ....
has been proposed as a method to remove this greenhouse gas
Greenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
from the atmosphere and control climate change
Climate change
Climate change is a significant and lasting change in the statistical distribution of weather patterns over periods ranging from decades to millions of years. It may be a change in average weather conditions or the distribution of events around that average...
.
Clathrates are suspected to occur in large quantities on some outer planet
Planet
A planet is a celestial body orbiting a star or stellar remnant that is massive enough to be rounded by its own gravity, is not massive enough to cause thermonuclear fusion, and has cleared its neighbouring region of planetesimals.The term planet is ancient, with ties to history, science,...
s, moons
Natural satellite
A natural satellite or moon is a celestial body that orbits a planet or smaller body, which is called its primary. The two terms are used synonymously for non-artificial satellites of planets, of dwarf planets, and of minor planets....
and trans-Neptunian object
Trans-Neptunian object
A trans-Neptunian object is any minor planet in the Solar System that orbits the Sun at a greater distance on average than Neptune.The first trans-Neptunian object to be discovered was Pluto in 1930...
s, binding gas at fairly high temperatures.
Structure

Crystallography
Crystallography is the experimental science of the arrangement of atoms in solids. The word "crystallography" derives from the Greek words crystallon = cold drop / frozen drop, with its meaning extending to all solids with some degree of transparency, and grapho = write.Before the development of...
cubic structures – structure (Type) I and structure (Type) II of space groups
 and
and  respectively. Seldom, a third hexagonal structure of space group
respectively. Seldom, a third hexagonal structure of space group  may be observed (Type H).
may be observed (Type H).The unit cell of Type I consists of 46 water molecules, forming two types of cages – small and large. The small cages in the unit cell are two against six large ones. The small cage has the shape of a pentagonal dodecahedron (512) and the large one that of a tetradecahedron
Tetradecahedron
A tetradecahedron is a polyhedron with 14 faces. There are numerous topologically distinct forms of a tetradecahedron, with many constructible entirely with regular polygon faces....
, specifically a hexagonal truncated trapezohedron
Hexagonal truncated trapezohedron
The hexagonal truncated trapezohedron is the fourth in an infinite series of truncated trapezohedron polyhedra. It has 12 pentagon and 2 hexagon faces....
(51262), together forming a Weaire-Phelan structure
Weaire-Phelan structure
In geometry, the Weaire–Phelan structure is a complex 3-dimensional structure representing an idealised foam of equal-sized bubbles. In 1993, Trinity College Dublin physicist Denis Weaire and his student Robert Phelan found that in computer simulations of foam, this structure was a better...
. Typical guests forming Type I hydrates are CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
in carbon dioxide clathrate
Carbon dioxide clathrate
Carbon dioxide hydrate is a Type I gas clathrate . However, there has been some experimental evidence for the development of a metastable Type II phase at temperature near the ice melting point ....
and CH4
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
in methane clathrate
Methane clathrate
Methane clathrate, also called methane hydrate, hydromethane, methane ice, "fire ice", natural gas hydrate or just gas hydrate, is a solid clathrate compound in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice...
.
The unit cell of Type II consists of 136 water molecules, forming also two types of cages – small and large. In this case the small cages in the unit cell are sixteen against eight large ones. The small cage has again the shape of a pentagonal dodecahedron (512) but the large one is a hexadecahedron (51264). Type II hydrates are formed by gases like O2 and N2.
The unit cell of Type H consists of 34 water molecules, forming three types of cages – two small of different type and one huge. In this case, the unit cell consists of three small cages of type 512, twelve small ones of type 435663 and one huge of type 51268. The formation of Type H requires the cooperation of two guest gases (large and small) to be stable. It is the large cavity that allows structure H hydrates to fit in large molecules (e.g. butane
Butane
Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or...
, hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s), given the presence of other smaller help gases to fill and support the remaining cavities. Structure H hydrates were suggested to exist in the Gulf of Mexico. Thermogenically-produced supplies of heavy hydrocarbons are common there.
Hydrates in the Universe
Iro et al., trying to interpret the nitrogenNitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
deficiency in comet
Comet
A comet is an icy small Solar System body that, when close enough to the Sun, displays a visible coma and sometimes also a tail. These phenomena are both due to the effects of solar radiation and the solar wind upon the nucleus of the comet...
s, stated most of the conditions for hydrate formation in the protoplanetary nebulae
Protoplanetary disk
A protoplanetary disk is a rotating circumstellar disk of dense gas surrounding a young newly formed star, a T Tauri star, or Herbig Ae/Be star...
, surrounding the pre-main and main sequence
Main sequence
The main sequence is a continuous and distinctive band of stars that appears on plots of stellar color versus brightness. These color-magnitude plots are known as Hertzsprung–Russell diagrams after their co-developers, Ejnar Hertzsprung and Henry Norris Russell...
stars were fulfilled, despite the rapid grain growth to meter scale. The key was to provide enough microscopic ice particles exposed to a gaseous environment. Observations of the radiometric
Radiometry
In optics, radiometry is a set of techniques for measuring electromagnetic radiation, including visible light. Radiometric techniques characterize the distribution of the radiation's power in space, as opposed to photometric techniques, which characterize the light's interaction with the human eye...
continuum
Continuum (theory)
Continuum theories or models explain variation as involving a gradual quantitative transition without abrupt changes or discontinuities. It can be contrasted with 'categorical' models which propose qualitatively different states.-In physics:...
of circumstellar discs
Protoplanetary disk
A protoplanetary disk is a rotating circumstellar disk of dense gas surrounding a young newly formed star, a T Tauri star, or Herbig Ae/Be star...
around
 -Tauri
-TauriT Tauri star
T Tauri stars are a class of variable stars named after their prototype – T Tauri. They are found near molecular clouds and identified by their optical variability and strong chromospheric lines.-Characteristics:...
and Herbig Ae/Be stars suggest massive dust disks consisting of millimeter-sized grains, which disappear after several million years (e.g.,). A lot of work on detecting water ices in the Universe was done on the Infrared Space Observatory
Infrared Space Observatory
The Infrared Space Observatory was a space telescope for infrared light designed and operated by the European Space Agency , in cooperation with ISAS and NASA...
(ISO). For instance, broad emission bands
Spectral bands
Spectral bands are part of optical spectra of polyatomic systems, including condensed materials, large molecules etc. Each line corresponding to one level in atom splits in molecules. When the number of atoms is large, one gets continuum of energy levels, so called "spectral bands". They are often...
of water ice at 43 and 60 μm were found in the disk of the isolated Herbig Ae/Be star HD 100546 in Musca
Musca
Musca is one of the minor southern constellations. The constellation was one of twelve constellations created by Petrus Plancius from the observations of Pieter Dirkszoon Keyser and Frederick de Houtman and it first appeared on a 35-cm diameter celestial globe published in 1597 in Amsterdam by...
. The one at 43 μm is much weaker than the one at 60 μm, which means the water ice, is located in the outer parts of the disk at temperatures below 50 K. There is also another broad ice feature between 87 and 90 μm, which is very similar to the one in NGC 6302
NGC 6302
NGC 6302 is a bipolar planetary nebula in the constellation Scorpius. The structure in the nebula is among the most complex ever observed in planetary nebulae...
(the Bug or Butterfly nebula in Scorpius
Scorpius
Scorpius, sometimes known as Scorpio, is one of the constellations of the zodiac. Its name is Latin for scorpion, and its symbol is . It lies between Libra to the west and Sagittarius to the east...
). Crystalline ices were also detected in the proto-planetary disks of ε-Eridani
Epsilon Eridani
Epsilon Eridani is a star in the southern constellation Eridanus, along a declination 9.46° south of the celestial equator. This allows the star to be viewed from most of the Earth's surface. At a distance of 10.5 light years , it has an apparent magnitude of 3.73...
and the isolated Fe star HD 142527 in Lupus
Lupus (constellation)
Lupus is a constellation in the southern sky. Its name is Latin for wolf. Lupus was one of the 48 constellations listed by the 2nd century astronomer Ptolemy, and it remains one of the 88 modern constellations...
. 90 % of the ice in the latter was found crystalline at temperature around 50 K. HST
Hubble Space Telescope
The Hubble Space Telescope is a space telescope that was carried into orbit by a Space Shuttle in 1990 and remains in operation. A 2.4 meter aperture telescope in low Earth orbit, Hubble's four main instruments observe in the near ultraviolet, visible, and near infrared...
demonstrated that relatively old circumstellar disks
Protoplanetary disk
A protoplanetary disk is a rotating circumstellar disk of dense gas surrounding a young newly formed star, a T Tauri star, or Herbig Ae/Be star...
, as the one around the 5 million year old B9.5Ve Herbig Ae/Be star HD 141569A, are dusty. Li & Lunine found water ice there. Knowing the ices usually exist at the outer parts of the proto-planetary nebulae
Protoplanetary disk
A protoplanetary disk is a rotating circumstellar disk of dense gas surrounding a young newly formed star, a T Tauri star, or Herbig Ae/Be star...
, Hersant et al. proposed an interpretation of the volatile enrichment, observed in the four giant planets
Gas giant
A gas giant is a large planet that is not primarily composed of rock or other solid matter. There are four gas giants in the Solar System: Jupiter, Saturn, Uranus, and Neptune...
of the Solar System
Solar System
The Solar System consists of the Sun and the astronomical objects gravitationally bound in orbit around it, all of which formed from the collapse of a giant molecular cloud approximately 4.6 billion years ago. The vast majority of the system's mass is in the Sun...
, with respect to the Solar abundances
Abundance of the chemical elements
The abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...
. They assumed the volatiles had been trapped in the form of hydrates and incorporated in the planetesimal
Planetesimal
Planetesimals are solid objects thought to exist in protoplanetary disks and in debris disks.A widely accepted theory of planet formation, the so-called planetesimal hypothesis of Viktor Safronov, states that planets form out of cosmic dust grains that collide and stick to form larger and larger...
s flying in the protoplanets’
Protoplanet
Protoplanets are large planetary embryos that originate within protoplanetary discs and have undergone internal melting to produce differentiated interiors. They are believed to form out of kilometer-sized planetesimals that attract each other gravitationally and collide...
feeding zones.
Kieffer et al. (2006) suggest that the geyser activity in the south polar region of Saturn
Saturn
Saturn is the sixth planet from the Sun and the second largest planet in the Solar System, after Jupiter. Saturn is named after the Roman god Saturn, equated to the Greek Cronus , the Babylonian Ninurta and the Hindu Shani. Saturn's astronomical symbol represents the Roman god's sickle.Saturn,...
's moon Enceladus
Enceladus (moon)
Enceladus is the sixth-largest of the moons of Saturn. It was discovered in 1789 by William Herschel. Until the two Voyager spacecraft passed near it in the early 1980s very little was known about this small moon besides the identification of water ice on its surface...
originates from clathrate hydrates, where carbon dioxide, methane, and nitrogen are released when exposed to the vacuum of space by the "Tiger Stripe
Tiger Stripes (Enceladus)
The tiger stripes of Enceladus consist of four sub-parallel, linear depressions in the south polar region of the Saturnian moon. First observed on May 20, 2005 by the Cassini spacecraft's Imaging Science Sub-system camera , the features are most notable in lower resolution images by their...
" fractures found in that area.
Carbon dioxide clathrate
Carbon dioxide clathrate
Carbon dioxide hydrate is a Type I gas clathrate . However, there has been some experimental evidence for the development of a metastable Type II phase at temperature near the ice melting point ....
is believed to play a major role in different processes on Mars. Hydrogen clathrate
Hydrogen clathrate
A hydrogen clathrate is a clathrate containing hydrogen in water ice. This substance is interesting due to its possible use to store hydrogen in a hydrogen economy. Also interesting is that multiple hydrogen molecules can occur at each cage site in the ice. The maximum ration of hydrogen to water...
is likely to form in condensation nebulae for gas giants.
Natural gas hydrates
Naturally on EarthEarth
Earth is the third planet from the Sun, and the densest and fifth-largest of the eight planets in the Solar System. It is also the largest of the Solar System's four terrestrial planets...
gas hydrates can be found on the seafloor, in ocean sediments, in deep lake sediments (e.g. Lake Baikal
Lake Baikal
Lake Baikal is the world's oldest at 30 million years old and deepest lake with an average depth of 744.4 metres.Located in the south of the Russian region of Siberia, between Irkutsk Oblast to the northwest and the Buryat Republic to the southeast, it is the most voluminous freshwater lake in the...
), as well as in the permafrost
Permafrost
In geology, permafrost, cryotic soil or permafrost soil is soil at or below the freezing point of water for two or more years. Ice is not always present, as may be in the case of nonporous bedrock, but it frequently occurs and it may be in amounts exceeding the potential hydraulic saturation of...
regions. The amount of methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
potentially trapped in natural methane hydrate
Methane clathrate
Methane clathrate, also called methane hydrate, hydromethane, methane ice, "fire ice", natural gas hydrate or just gas hydrate, is a solid clathrate compound in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice...
deposits may be significant (1015 to 1017 cubic metres), which makes them of major interest as a potential energy resource. Catastrophic release of methane from the decomposition of such deposits may lead to a global climate change, because CH
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
4 is more efficient greenhouse gas even than CO
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
2 (see Atmospheric methane
Atmospheric methane
Atmospheric methane levels are of interest due to its impact on climate change. Atmospheric methane is one of the most potent and influential greenhouse gases on Earth. The 100-year global warming potential of methane is 25, i.e...
). The fast decomposition of such deposits is considered a geohazard
Geohazard
A geohazard can be defined as a geological state that represents or has the potential to develop further into a situation leading to damage or uncontrolled risk. This definition implies that geohazards are widespread phenomena that are related to geological and environmental conditions and involve...
, due to its potential to trigger landslide
Landslide
A landslide or landslip is a geological phenomenon which includes a wide range of ground movement, such as rockfalls, deep failure of slopes and shallow debris flows, which can occur in offshore, coastal and onshore environments...
s, earthquake
Earthquake
An earthquake is the result of a sudden release of energy in the Earth's crust that creates seismic waves. The seismicity, seismism or seismic activity of an area refers to the frequency, type and size of earthquakes experienced over a period of time...
s and tsunami
Tsunami
A tsunami is a series of water waves caused by the displacement of a large volume of a body of water, typically an ocean or a large lake...
s. However, natural gas hydrates do not contain only methane but also other hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
gases, as well as H2S
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
and CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
. Air hydrates are frequently observed in polar ice samples.
Pingo
Pingo
A pingo, also called a hydrolaccolith, is a mound of earth-covered ice found in the Arctic and subarctic that can reach up to in height and up to in diameter. The term originated as the Inuvialuktun word for a small hill. A pingo is a periglacial landform, which is defined as a nonglacial...
s are common structures in permafrost regions. Similar structures are found in deep water related to methane leakages.
It is important to notice that gas hydrates can even be formed in the absence of a liquid phase. Under that situation, water is dissolved in gas or in liquid hydrocarbon phase .
Gas hydrates in pipelines
Thermodynamic conditions favouring hydrate formation are often found in pipelinesPipeline transport
Pipeline transport is the transportation of goods through a pipe. Most commonly, liquids and gases are sent, but pneumatic tubes that transport solid capsules using compressed air are also used....
. This is highly undesirable because the clathrate crystals might agglomerate and plug the flowline and cause flow assurance
Flow assurance
Flow assurance is a relatively new term in oil and gas industry. It refers to ensuring successful and economical flow of hydrocarbon stream from reservoir to the point of sale...
failure and damage valves and instrumentation. The results can range from flow reduction to equipment damage.
Hydrate formation, prevention and mitigation philosophy
Hydrates have a strong tendency to agglomerateAgglomerate
Agglomerates are coarse accumulations of large blocks of volcanic material that contain at least 75% bombs...
and to adhere to the pipe wall and thereby plug the pipeline. Once formed, they can be decomposed by increasing the temperature and/or decreasing the pressure. Even under these conditions, the clathrate dissociation is a slow process.
Therefore, preventing hydrate formation appears to be the key to the problem. A hydrate prevention philosophy could typically be based on three levels of security, listed in order of priority:
- Avoid operational conditions that might cause formation of hydrates by depressing the hydrate formation temperature using glycol dehydrationGlycol dehydrationGlycol dehydration is a liquid desiccant system for the removal of water from natural gas and natural gas liquids . It is the most common and economic means of water removal from these streams. Glycols typically seen in industry include triethylene glycol , diethylene glycol , ethylene glycol ,...
; - Temporarily change operating conditions in order to avoid hydrate formation;
- Prevent formation of hydrates by addition of chemicals that (a) shift the hydrate equilibrium conditions towards lower temperatures and higher pressures or (b) increase hydrate formation time (inhibitorReaction inhibitorA reaction inhibitor is a substance that decreases the rate of, or prevents, a chemical reaction.-Inhibition of a catalyst:An inhibitor can reduce the effectiveness of a catalyst in a catalysed reaction...
s)
The actual philosophy would depend on operational circumstances such as pressure, temperature, type of flow (gas, liquid, presences of water etc.)
Hydrate inhibitors
When operating within a set of parameters where hydrates could be formed, there are still ways to avoid their formation. Altering the gas composition by adding chemicals can lower the hydrate formation temperature and/or delay their formation. Two options generally exist:- Thermodynamic inhibitors
- Kinetic inhibitors/anti-agglomerants
The most common thermodynamic inhibitors are, methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, monoethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
(MEG) and diethylene glycol
Diethylene glycol
Diethylene glycol is an organic compound with the formula 2O. It is a colorless, practically odorless, poisonous, and hygroscopic liquid with a sweetish taste. It is miscible in water, alcohol, ether, acetone, and ethylene glycol. DEG is a widely used solvent...
(DEG) commonly referred to as glycol. All may be recovered and recirculated, but the economics of methanol recovery is not favourable in most cases. MEG is preferred over DEG for applications where the temperature is expected to be −10 °C or lower due to high viscosity at low temperatures. Triethylene glycol
Triethylene glycol
Triethylene glycol, TEG, or triglycol is a colorless odorless viscous liquid with molecular formula HOCH2CH2OCH2CH2OCH2CH2OH. It is used as a plasticizer for vinyl. It is also used in air sanitizer products, such as "Oust" or "Clean and Pure." When aerosolized it acts as a disinfectant...
(TEG) has too low vapour pressure to be suited as an inhibitor injected into a gas stream. More methanol is lost in the gas phase when compared to MEG or DEG.
The use of kinetic inhibitors and anti-agglomerants in actual field operations is a new and evolving technology. It requires extensive tests and optimisation to the actual system. While kinetic inhibitors work by slowing down the kinetics of the nucleation, anti-agglomerants do not stop the nucleation, they rather stop the agglomeration (sticking together) of gas hydrate crystals. These two kinds of inhibitors are also known as Low-Dosage-Hydrate-Inhibitors because they require much smaller concentrations than the conventional thermodynamic inhibitors. Kinetic inhibitors (which do not require water and hydrocarbon mixture to be effective) are usually polymers or copolymers and anti-agglomerants (requires water and hydrocarbon mixture) are polymers or zwitterionic (usually ammonium and COOH) surfactants being both attracted to hydrates and hydrocarbons.
See also
- Clathrate
- Ice core brittle zone
- Star Formation and evolution
- Clathrate gun hypothesisClathrate gun hypothesisThe clathrate gun hypothesis is the popular name given to the hypothesis that rises in sea temperatures can trigger the sudden release of methane from methane clathrate compounds buried in seabeds and permafrost which, because the methane itself is a powerful greenhouse gas, leads to further...
Further reading
- Shuqiang Gao, Waylon House, and Walter Chapman, “NMR/MRI Study of Clathrate Hydrate Mechanisms”, J. Phys. Chem. B, 109(41), 19090-19093, 2005.
- http://gashydrate.fileave.com/NMR-MRI%20study%20of%20clathrate%20hydrate%20mechanisms.pdf
- Mohamed Iqbal Pallipurath, "DISSOCIATION OF HYDRATED MARINE SEDIMENT", Oil and Gas Business Journal, 2006 http://www.ogbus.ru/eng/authors/Iqbal/Iqbal_1.pdf
- N Sultan, P Cochonat, JP Foucher, J Mienert, Effect of gas hydrates melting on seafloor slope instability - ►ifremer.fr [PDF], - Marine Geology, 2004 - Elsevier http://linkinghub.elsevier.com/retrieve/pii/S0025322704002798
External links
- Gas hydrates, from Leibniz Institute of Marine Sciences, Kiel (IFM-GEOMAR)
- The SUGAR Project (Submarine Gas Hydrate Reservoirs), from Leibniz Institute of Marine Sciences, Kiel (IFM-GEOMAR)
- Gas hydrates in video and - Background knowledge about gas hydrates, their prevention and removal (by manufacturer of hydrate autoclaves)

