
Coiled coil
Encyclopedia
Structural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
in protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, in which 2-7 alpha-helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
are coiled together like the strands of a rope (dimer
Protein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
s and trimers are the most common types). Many coiled coil type proteins are involved in important biological functions such as the regulation of gene expression
Gene expression
Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product. These products are often proteins, but in non-protein coding genes such as ribosomal RNA , transfer RNA or small nuclear RNA genes, the product is a functional RNA...
e.g. transcription factor
Transcription factor
In molecular biology and genetics, a transcription factor is a protein that binds to specific DNA sequences, thereby controlling the flow of genetic information from DNA to mRNA...
s. Notable examples are the oncoproteins c-fos and jun, and the muscle protein tropomyosin
Tropomyosin
Tropomyosin is an actin-binding protein that regulates actin mechanics. It is important, among other things, for muscle contraction. Tropomyosin, along with the troponin complex, associate with actin in muscle fibers and regulate muscle contraction by regulating the binding of myosin...
.
Discovery of coiled coils
The possibility of coiled coils for α-keratinKeratin
Keratin refers to a family of fibrous structural proteins. Keratin is the key of structural material making up the outer layer of human skin. It is also the key structural component of hair and nails...
was proposed by Francis Crick
Francis Crick
Francis Harry Compton Crick OM FRS was an English molecular biologist, biophysicist, and neuroscientist, and most noted for being one of two co-discoverers of the structure of the DNA molecule in 1953, together with James D. Watson...
in 1952 as well as mathematical methods for determining their structure. Remarkably, this was soon after the structure of the alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
was suggested in 1951 by Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
and coworkers.
Molecular structure of coiled coils
Coiled coils usually contain a repeated pattern, hxxhcxc, of hydrophobic (h) and charged (c) amino-acid residues, referred to as a heptad repeatHeptad repeat
The heptad repeat is an example of a structural motif that consists of a repeating pattern of seven amino acids: a b c d e f g H P P H C P C...
.
The positions in the heptad repeat are usually labeled abcdefg, where a and d are the hydrophobic positions, often being occupied by isoleucine
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
, leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
or valine
Valine
Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar...
. Folding a sequence with this repeating pattern into an alpha-helical secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
causes the hydrophobic residues to be presented as a 'stripe' that coils gently around the helix in left-handed fashion, forming an amphipathic structure. The most favorable way for two such helices to arrange themselves in the water-filled environment of the cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
is to wrap the hydrophobic strands against each other sandwiched between the hydrophilic amino acids. It is thus the burial of hydrophobic surfaces, that provides the thermodynamic driving force for the oligomerization. The packing in a coiled-coil interface is exceptionally tight, with almost complete van der Waals
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
contact between the side chains of the a and d residues. This tight packing was originally predicted by Francis Crick
Francis Crick
Francis Harry Compton Crick OM FRS was an English molecular biologist, biophysicist, and neuroscientist, and most noted for being one of two co-discoverers of the structure of the DNA molecule in 1953, together with James D. Watson...
in 1952 and is referred to as Knobs into holes packing
Knobs into holes packing
Knobs into holes packing is a protein packing motif that occurs mainly in alpha helix or coiled coil domains. One such example is fibrinogen fibril formation....
.
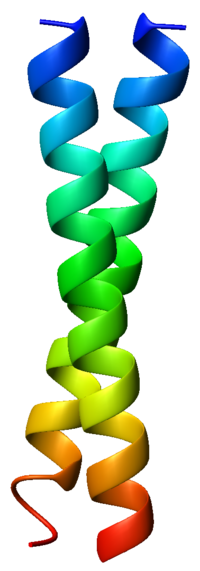
The α-helices may be parallel or anti-parallel, and usually adopt a left-handed super-coil (Figure 1). Although disfavored, a few right-handed coiled coils have also been observed in nature and in designed proteins.
Role of coiled coils in HIV infection
Gp120
Envelope glycoprotein GP120 is a glycoprotein exposed on the surface of the HIV envelope. The 120 in its name comes from its molecular weight of 120 kilodaltons...
) bind to CD4 receptor and a coreceptor. gp120 is closely associated to a trimer of gp41 via van der Waals interactions. Upon binding of gp120 to the CD4 receptor and coreceptor, a number of conformational changes in the structure leads to the dissociation of gp120 and to the exposure of gp41
Gp41
gp41 is a subunit of the envelope protein complex of retroviruses, including Human immunodeficiency virus and Simian-Human immunodeficiency virus. This glycoprotein subunit remains non-covalently-bound to gp120, and provides the second step by which HIV enters the cell...
and at the same time to the fusion peptide sequence located at the N-terminus of gp41 to anchor into the host cell. A spring-loaded mechanism is responsible for bringing the viral and cell membranes in close proximity that they will fuse. The origin of the spring-loaded mechanism lies within the exposed gp41
Gp41
gp41 is a subunit of the envelope protein complex of retroviruses, including Human immunodeficiency virus and Simian-Human immunodeficiency virus. This glycoprotein subunit remains non-covalently-bound to gp120, and provides the second step by which HIV enters the cell...
, which has two heptad repeat repeats located at the N-terminal (HR1) as well as the C-terminal (HR2) side of the protein. HR1 forms a parallel, trimeric coiled coil on to which HR2 region coils forming the trimer-of-hairpins (or six-helix bundle) structure thereby facilitating membrane fusion through bringing the membranes close to each other. The virus then enters the cell and begins its replication. Recently, inhibitors derived from HR2 such as Fuzeon
Enfuvirtide
Enfuvirtide is an HIV fusion inhibitor, the first of a novel class of antiretroviral drugs used in combination therapy for the treatment of HIV-1 infection. It is marketed under the trade name Fuzeon ....
(DP178, T-20) bind to the HR1 region on gp41 have been developed. Conversely, peptides derived from HR1 have little viral inhibition efficacy due to the propensity for these peptides to aggregate in solution. Chimeras of these HR1 derived peptides with GCN4 leucine zipper
Leucine zipper
A leucine zipper, aka leucine scissors, is a common three-dimensional structural motif in proteins. These motifs are usually found as part of a DNA-binding domain in various transcription factors, and are therefore involved in regulating gene expression...
s have been developed and have shown to be more active than Fuzeon
Enfuvirtide
Enfuvirtide is an HIV fusion inhibitor, the first of a novel class of antiretroviral drugs used in combination therapy for the treatment of HIV-1 infection. It is marketed under the trade name Fuzeon ....
, but these have not entered the clinic yet.
Coiled coils as dimerization tags
Because of their specific interaction coiled coils can be used as a dimerization "tag".Coiled-coil Design
The general problem of deciding on the folded structure of a protein when given the amino acid sequence (the so-called protein folding problemProtein structure prediction
Protein structure prediction is the prediction of the three-dimensional structure of a protein from its amino acid sequence — that is, the prediction of its secondary, tertiary, and quaternary structure from its primary structure. Structure prediction is fundamentally different from the inverse...
) has not been solved. However, the coiled coil is one of a relatively small number of folding motifs for which the relationships between the sequence and the final folded structure are comparatively well-understood. Harbury et al.. performed a landmark study using an archetypal coiled coil, GCN4, in which rules were established that govern the way that peptide sequence affects the oligomeric state (that is, the number of alpha-helices in the final assembly). The GCN4 coiled coil is a 31-amino-acid (which equates to just over four heptads) parallel, dimeric (i.e., consisting of two alpha-helices) coiled coil and has a repeated isoleucine
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
(or I, in single-letter code) and leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
(L) at the a and d positions, respectively, and forms a dimeric coiled coil. When the amino acids in the a and d positions were changed from I at a and L at d to L at a and I at d, a trimeric (three alpha-helices) coiled coil was formed. Furthermore, mutating the a and d positions both to L resulted in the formation of a tetrameric (four alpha-helices) coiled coil. These represent an astonishingly simple set of rules for the determination of coiled coil oligomeric states and allows scientists to effectively "dial-in" the oligomerization behavior. Another aspect of coiled coil assembly that is relatively well understood, at least in the case of dimeric coiled coils, is that placing a polar residue (in particular asparagine
Asparagine
Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
, N) at opposing a positions forces parallel assembly of the coiled coil. This effect is due to a self-complementary hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing between these residues, which would go unsatisfied if an N was paired with, for instance, an L on the opposing helix.
Prediction and detection
- JCoils predict Coiled Coil in a protein sequence
- NCOILS
- Paircoil2 / Paircoil
- bCIPA
- STRAP contains an algorithm to predict coiled-coils from AA-sequences
- Socket is a program that is able to identify coiled coils in proteins from atomic structures derived from crystallographic or NMRProtein nuclear magnetic resonance spectroscopyNuclear magnetic resonance spectroscopy of proteins is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins. The field was pioneered by Richard R. Ernst and Kurt Wüthrich, among others...
studies. - PrOCoil predicts the oligomerization of coiled coil proteins and visualizes the contribution of each individual amino acid to the overall oligomeric tendency.
Databases
- CC+ is a relational databaseRelational databaseA relational database is a database that conforms to relational model theory. The software used in a relational database is called a relational database management system . Colloquial use of the term "relational database" may refer to the RDBMS software, or the relational database itself...
of coiled coils found in the PDBProtein Data BankThe Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids.... - SUPERFAMILY protein domain annotation for all completely sequenced organisms based on the expertly curated SCOPStructural Classification of ProteinsThe Structural Classification of Proteins database is a largely manual classification of protein structural domains based on similarities of their structures and amino acid sequences. A motivation for this classification is to determine the evolutionary relationship between proteins...
coiled coil class

