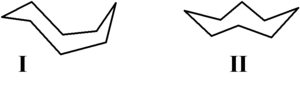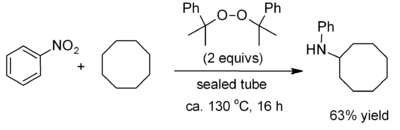Cyclooctane
Encyclopedia
Cyclooctane is a cycloalkane
with the molecular formula (CH2)8. It is a simple colourless hydrocarbon
, but it is often a reference compound for saturated eight-membered ring compounds in general.
methods. Hendrickson noted that "cyclooctane is unquestionably the conformationally most complex cycloalkane owing to the existence of many conformers of comparable energy." The boat-chair conformation I is the most stable form. This conformation was confirmed by Allinger and co-workers. The crown conformation II is slightly less stable. Among the many compounds exhibiting the crown conformation (structure II) is S8, elemental sulfur
.
(COD), which can be hydrogenated. COD is widely used for the preparation of precatalysts for homogeneous catalysis
. The activation of these catalysts under H2, produces cyclooctane, which is usually discarded or burnt:
Cyclooctane participates in no reactions except those typical of a other saturated hydrocarbons, combustion
and free radical halogenation
. Recent work on alkane functionalisation, using peroxides such as dicumyl peroxide, has opened up the chemistry to some extent, allowing for example the introduction of a phenylamino group.
Cycloalkane
Cycloalkanes are types of alkanes that have one or more rings of carbon atoms in the chemical structure of their molecules. Alkanes are types of organic hydrocarbon compounds that have only single chemical bonds in their chemical structure...
with the molecular formula (CH2)8. It is a simple colourless hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
, but it is often a reference compound for saturated eight-membered ring compounds in general.
Conformation
The conformation has been studied extensively using computationalComputational chemistry
Computational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
methods. Hendrickson noted that "cyclooctane is unquestionably the conformationally most complex cycloalkane owing to the existence of many conformers of comparable energy." The boat-chair conformation I is the most stable form. This conformation was confirmed by Allinger and co-workers. The crown conformation II is slightly less stable. Among the many compounds exhibiting the crown conformation (structure II) is S8, elemental sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
.
Synthesis and reactions
The main route to cyclooctane derivatives involves the dimerization of butadiene, catalysed by nickel(0) complexes such as nickel bis(cyclooctadiene). This process affords, among other products, 1,5-cyclooctadieneCyclooctadiene
A cyclooctadiene is a cyclic diene with the formula C8H12. Focusing only on cis derivatives, four isomers are possible: 1,2, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers are 1,3-cyclooctadiene and 1,5-cyclooctadiene, which is used as a ligand for transition...
(COD), which can be hydrogenated. COD is widely used for the preparation of precatalysts for homogeneous catalysis
Homogeneous catalysis
In chemistry, homogeneous catalysis is a sequence of reactions that involve a catalyst in the same phase as the reactants. Most commonly, a homogeneous catalyst is codissolved in a solvent with the reactants.-Acid catalysis:...
. The activation of these catalysts under H2, produces cyclooctane, which is usually discarded or burnt:
- C8H12 + 2 H2 → C8H16
Cyclooctane participates in no reactions except those typical of a other saturated hydrocarbons, combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
and free radical halogenation
Free radical halogenation
In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of heat or UV light. The reaction is used for the industrial synthesis of chloroform , dichloromethane , and hexachlorobutadiene...
. Recent work on alkane functionalisation, using peroxides such as dicumyl peroxide, has opened up the chemistry to some extent, allowing for example the introduction of a phenylamino group.