
Dielectric spectroscopy
Encyclopedia
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
(sometimes called impedance
Electrical impedance
Electrical impedance, or simply impedance, is the measure of the opposition that an electrical circuit presents to the passage of a current when a voltage is applied. In quantitative terms, it is the complex ratio of the voltage to the current in an alternating current circuit...
spectroscopy), and also known as electrochemical impedance spectroscopy, measures the dielectric
Dielectric
A dielectric is an electrical insulator that can be polarized by an applied electric field. When a dielectric is placed in an electric field, electric charges do not flow through the material, as in a conductor, but only slightly shift from their average equilibrium positions causing dielectric...
properties of a medium as a function of frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
. It is based on the interaction of an external field with the electric dipole moment
Electric dipole moment
In physics, the electric dipole moment is a measure of the separation of positive and negative electrical charges in a system of charges, that is, a measure of the charge system's overall polarity with SI units of Coulomb-meter...
of the sample, often expressed by permittivity
Permittivity
In electromagnetism, absolute permittivity is the measure of the resistance that is encountered when forming an electric field in a medium. In other words, permittivity is a measure of how an electric field affects, and is affected by, a dielectric medium. The permittivity of a medium describes how...
.
It is also an experimental method of characterizing electrochemical systems. This technique measures the impedance
Electrical impedance
Electrical impedance, or simply impedance, is the measure of the opposition that an electrical circuit presents to the passage of a current when a voltage is applied. In quantitative terms, it is the complex ratio of the voltage to the current in an alternating current circuit...
of a system over a range of frequencies, and therefore the frequency response of the system, including the energy storage and dissipation properties, is revealed. Often, data obtained by EIS is expressed graphically in a Bode plot
Bode plot
A Bode plot is a graph of the transfer function of a linear, time-invariant system versus frequency, plotted with a log-frequency axis, to show the system's frequency response...
or a Nyquist plot
Nyquist plot
A Nyquist plot is a parametric plot of a transfer function used in automatic control and signal processing. The most common use of Nyquist plots is for assessing the stability of a system with feedback. In Cartesian coordinates, the real part of the transfer function is plotted on the X axis. The...
.
Impedance is the opposition to the flow of alternating current
Alternating current
In alternating current the movement of electric charge periodically reverses direction. In direct current , the flow of electric charge is only in one direction....
(AC) in a complex system. A passive complex electrical system comprises both energy dissipater (resistor
Resistor
A linear resistor is a linear, passive two-terminal electrical component that implements electrical resistance as a circuit element.The current through a resistor is in direct proportion to the voltage across the resistor's terminals. Thus, the ratio of the voltage applied across a resistor's...
) and energy storage (capacitor
Capacitor
A capacitor is a passive two-terminal electrical component used to store energy in an electric field. The forms of practical capacitors vary widely, but all contain at least two electrical conductors separated by a dielectric ; for example, one common construction consists of metal foils separated...
) elements. If the system is purely resistive, then the opposition to AC or direct current
Direct current
Direct current is the unidirectional flow of electric charge. Direct current is produced by such sources as batteries, thermocouples, solar cells, and commutator-type electric machines of the dynamo type. Direct current may flow in a conductor such as a wire, but can also flow through...
(DC) is simply resistance
Electrical resistance
The electrical resistance of an electrical element is the opposition to the passage of an electric current through that element; the inverse quantity is electrical conductance, the ease at which an electric current passes. Electrical resistance shares some conceptual parallels with the mechanical...
.
Almost any physico-chemical system, such as electrochemical cell
Electrochemical cell
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
s, mass-beam oscillators, and even biological tissue possesses energy storage and dissipation properties. EIS examines them.
This technique has grown tremendously in stature over the past few years and is now being widely employed in a wide variety of scientific fields such as fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
testing, biomolecular interaction, and microstructural characterization. Often, EIS reveals information about the reaction mechanism of an electrochemical process: different reaction steps will dominate at certain frequencies, and the frequency response shown by EIS can help identify the rate limiting step.
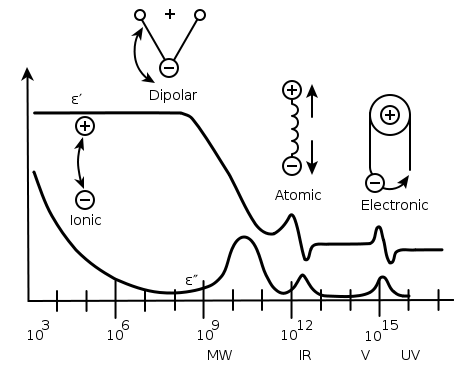
Dielectric mechanisms
There are a number of different dielectric mechanisms, connected to the way a studied medium reacts to the applied field (see the figure illustration). Each dielectric mechanism is centered around its characteristic frequency, which is the reciprocal of the characteristic timeCharacteristic time
The characteristic time is an estimate of the order of magnitude of the reaction time scale. It can loosely be defined as the inverse of the reaction rate...
of the process. In general, dielectric mechanisms can be divided into relaxation and resonance
Resonance
In physics, resonance is the tendency of a system to oscillate at a greater amplitude at some frequencies than at others. These are known as the system's resonant frequencies...
processes. The most common, starting from high frequencies, are:
Electronic polarization
This resonant process occurs in a neutral atomAtom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
when the electric field displaces the electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
relative to the nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
it surrounds.
This displacement occurs due to the equilibrium between restoration and electric forces.
Electronic polarization may be understood by assuming an atom as a point nucleus surrounded by spherical electron cloud of uniform charge density.
Atomic polarization
Atomic polarization is observed when the electronic cloud is deformed under the force of the applied field, so that the negative and positive charge are formed. This is a resonant process.Dipole relaxation
This originates from permanent and induced dipoleDipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
s aligning to an electric field. Their orientation polarisation is disturbed by thermal noise (which mis-aligns the dipole vectors from the direction of the field), and the time needed for dipoles to relax is determined by the local viscosity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
. These two facts make dipole relaxation heavily dependent on temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and chemical surrounding.
Ionic relaxation
Ionic relaxation comprises ionic conductivityIonic conductivity
Ionic conduction is the movement of an ion from one site to another through defects in the crystal lattice of a solid. Ionic conduction is one aspect of current....
and interfacial and space charge relaxation. Ionic conductivity predominates at low frequencies and introduces only losses to the system. Interfacial relaxation occurs when charge carriers are trapped at interfaces of heterogeneous systems. A related effect is Maxwell-Wagner-Sillars polarization
Maxwell-Wagner-Sillars polarization
In dielectric spectroscopy, large frequency dependent contributions to the dielectric response, especially at low frequencies, may come from build-ups of charge...
, where charge carriers blocked at inner dielectric boundary layers (on the mesoscopic scale) or external electrodes (on a macroscopic scale) lead to a separation of charges. The charges may be separated by a considerable distance and therefore make contributions to the dielectric loss that are orders of magnitude larger than the response due to molecular fluctuations.
Dielectric relaxation
Dielectric relaxation as a whole is the result of the movement of dipoles (dipole relaxation) and electric charges (ionic relaxation) due to an applied alternating field, and is usually observed in the frequency range 102-1010 Hz. Relaxation mechanisms are relatively slow compared to resonant electronic transitions or molecular vibrations, which usually have frequencies above 1012 Hz.Steady-state
For a redox reactionR
 O + e, without mass-transfer limitation, the relationship between the current density and the electrode overpotential is given by the Butler-Volmer equation:
O + e, without mass-transfer limitation, the relationship between the current density and the electrode overpotential is given by the Butler-Volmer equation:
with
 .
. is the exchange current density and
is the exchange current density and  and
and  are the symmetry factors.
are the symmetry factors.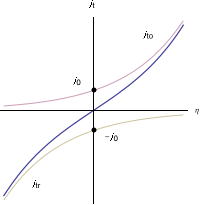
 is not a straight line (Fig. 1), therefore a redox reaction is not a linear system.
is not a straight line (Fig. 1), therefore a redox reaction is not a linear system.Faradaic impedance
Let us suppose that the Butler-Volmer relationship correctly describes the dynamic behavior of the redox reaction :
Dynamic behavior of the redox reaction is characterized by the so-called charge transfer resistance defined by :

The value of the charge transfer resistance changes with the overpotential. For this simplest example the Faradaic impedance is reduced to a resistance. It is worthwhile to notice that:

for
 .
.Double layer capacitance
An electrode electrolyte interface behaves like a capacitance called electrochemical double-layer capacitance
electrolyte interface behaves like a capacitance called electrochemical double-layer capacitance  . The equivalent electrical circuit for the redox reaction taking account of the double-layer capacitance is shown in Fig. 2. Another analog circuit commonly used to model the electrochemical double-layer is called a constant phase element
. The equivalent electrical circuit for the redox reaction taking account of the double-layer capacitance is shown in Fig. 2. Another analog circuit commonly used to model the electrochemical double-layer is called a constant phase elementConstant phase element
A constant phase element is an equivalent electrical circuit component that models the behaviour of a double layer, that is an imperfect capacitor....
.
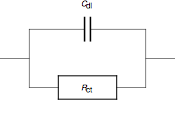

where
 is the angular frequency of a sinusoidal signal (rd/s), and
is the angular frequency of a sinusoidal signal (rd/s), and  .
.It is obtained :

Nyquist diagram of the impedance of the circuit shown in Fig. 3 is a semicircle with a diameter
 and an angular frequency at the apex equal to
and an angular frequency at the apex equal to  (Fig. 3). Others representations, Bode
(Fig. 3). Others representations, BodeBode plot
A Bode plot is a graph of the transfer function of a linear, time-invariant system versus frequency, plotted with a log-frequency axis, to show the system's frequency response...
or Black plans can be used.
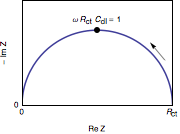
Ohmic resistance
The ohmic resistance appears in series with the electrode impedance of the reaction and the Nyquist diagram is translated to the right.
appears in series with the electrode impedance of the reaction and the Nyquist diagram is translated to the right.Measurement of the impedance parameters
Plotting the Nyquist diagram with a potentiostatPotentiostat
A potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments. A Bipotentiostat and polypotentiostat are potentiostats capable of controlling two working electrodes and more than two working electrodes, respectively.The system...
and an impedance analyzer, most often included in modern potentiostats, allows the user to determine charge transfer resistance, double layer capacitance and ohmic resistance. The exchange current density
 can be easily determined measuring the impedance of a redox reaction for
can be easily determined measuring the impedance of a redox reaction for  .
.Nyquist diagrams are made of several arcs for reaction more complex than redox reaction and with mass-transfer limitation.
See also
- loss tangentLoss tangentThe loss tangent is a parameter of a dielectric material that quantifies its inherent dissipation of electromagnetic energy. The term refers to the tangent of the angle in a complex plane between the resistive component of an electromagnetic field and its reactive component.-Electromagnetic...
- Debye relaxation
- EllipsometryEllipsometryEllipsometry is an optical technique for the investigation of the dielectric properties of thin films....
- Linear response functionLinear response functionA linear response function describes the input-output relationship of a signal transducer such as a radio turning electromagnetic waves into music or a neuron turning synaptic input into a response...
- Kramers–Kronig relation
- Green–Kubo relations
- ElectrochemistryElectrochemistryElectrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
- PotentiostatPotentiostatA potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments. A Bipotentiostat and polypotentiostat are potentiostats capable of controlling two working electrodes and more than two working electrodes, respectively.The system...

