
Emission factor
Encyclopedia
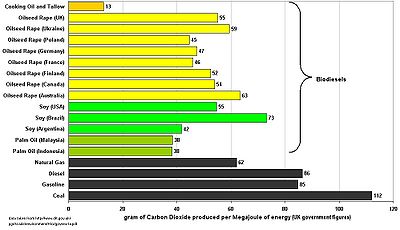
Pollutant
A pollutant is a waste material that pollutes air, water or soil, and is the cause of pollution.Three factors determine the severity of a pollutant: its chemical nature, its concentration and its persistence. Some pollutants are biodegradable and therefore will not persist in the environment in the...
from a given source relative to the intensity of a specific activity; for example gram
Gram
The gram is a metric system unit of mass....
s of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
released per megajoule of energy produced, or the ratio of greenhouse gas emissions produced to GDP. Emission intensities are used to derive estimates of air pollutant or greenhouse gas
Greenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
emissions based on the amount of fuel combusted
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
, the number of animals in animal husbandry
Animal husbandry
Animal husbandry is the agricultural practice of breeding and raising livestock.- History :Animal husbandry has been practiced for thousands of years, since the first domestication of animals....
, on industrial production levels, distances traveled or similar activity data. Emission intensities may also be used to compare the environmental impact of different fuels or activities. The related terms emission factor and carbon intensity are often used interchangeably, but "factors" exclude aggregate activities such as GDP, and "carbon" excludes other pollutants.

Estimating emissions
Emission factors assume a linear relation between the intensity of the activity and the emission resulting from this activity:Emissionpollutant = Activity * Emission Factorpollutant
Intensities are also used in projecting possible future scenarios such as those used in the IPCC assessments, along with projected future changes in population, economic activity and energy technologies. The interrelations of these variables is treated under the so-called Kaya identity
Kaya identity
The Kaya identity is an equation relating factors that determine the level of human impact on climate, in the form of emissions of the greenhouse gas carbon dioxide. It states that total emission level can be expressed as the product of four inputs: population, GDP per capita, energy use per unit...
.
The level of uncertainty of the resulting estimates depends significantly on the source category and the pollutant. Some examples:
- Carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2) emissions from the combustion of fuel can be estimated with a high degree of certainty regardless of how the fuel is used as these emissions depend almost exclusively on the carbonCarbonCarbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
content of the fuel, which is generally known with a high degree of precision. The same is true for sulphur dioxide (SO2), since also sulphur contents of fuels are generally well known. Both carbon and sulphur are almost completey oxidized during combustion and all carbon and sulphur atoms in the fuel will be present in the flue gasFlue gasFlue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases from a fireplace, oven, furnace, boiler or steam generator. Quite often, the flue gas refers to the combustion exhaust gas produced at power plants...
es as CO2 and SO2 respectively. - In contrast, the levels of other air pollutants and non-CO2 greenhouse gas emissions from combustion depend on the precise technology applied when fuel is combusted. These emissions are basically caused by either incomplete combustion of a small fraction of the fuel (carbon monoxideCarbon monoxideCarbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, methaneMethaneMethane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
, non-methane volatile organic compoundsNMVOCNMVOC is the abbreviation for non-methane volatile organic compounds.It is a generic term for a large variety of chemically different compounds, like for example, benzene, ethanol, formaldehyde, cyclohexane, 1,1,1-trichloroethane or acetone....
) or by complicated chemical and physical processes during the combustion and in the smoke stack or tailpipe. Examples of these are particulatesParticulatesParticulates – also known as particulate matter , suspended particulate matter , fine particles, and soot – are tiny subdivisions of solid matter suspended in a gas or liquid. In contrast, aerosol refers to particles and/or liquid droplets and the gas together. Sources of particulate matter can be...
, NOx, a mixture of nitric oxideNitric oxideNitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
, NO, and nitrogen dioxideNitrogen dioxideNitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
, NO2). - Nitrous oxideNitrous oxideNitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
(N2O) emissions from agricultural soils are highly uncertain because they depend very much on both the exact conditions of the soil, the application of fertilizers and meteorologicalMeteorologyMeteorology is the interdisciplinary scientific study of the atmosphere. Studies in the field stretch back millennia, though significant progress in meteorology did not occur until the 18th century. The 19th century saw breakthroughs occur after observing networks developed across several countries...
conditions.
| Fuel/ Resource |
Thermal g(CO2-eq)/MJth |
Energy Intensity W·hth/W·he |
Electric g(CO2-eq)/kW·he |
|---|---|---|---|
| Coal Coal Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure... |
B:91.50–91.72 Br:94.33 88 |
B:2.62–2.85 Br:3.46 3.01 |
994B:863–941 Br:1,175 955 |
| Oil Oil An oil is any substance that is liquid at ambient temperatures and does not mix with water but may mix with other oils and organic solvents. This general definition includes vegetable oils, volatile essential oils, petrochemical oils, and synthetic oils.... |
73 | 893 | |
| Natural gas Natural gas Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural... |
cc:68.20 oc:68.40 51 |
cc:2.35 oc:3.05 |
664cc:577 oc:751 599 |
| Geothermal Power Geothermal power Geothermal energy is thermal energy generated and stored in the Earth. Thermal energy is the energy that determines the temperature of matter. Earth's geothermal energy originates from the original formation of the planet and from radioactive decay of minerals... |
3~ | 40TL0–1 TH91–122 |
|
| Uranium Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... Nuclear power Nuclear power Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity... |
WL0.18 WH0.20 |
WL60 WH65 |
|
| Hydroelectricity Hydroelectricity Hydroelectricity is the term referring to electricity generated by hydropower; the production of electrical power through the use of the gravitational force of falling or flowing water. It is the most widely used form of renewable energy... |
15 | ||
| Conc. Solar Pwr | 40±15# | ||
| Photovoltaics Photovoltaics Photovoltaics is a method of generating electrical power by converting solar radiation into direct current electricity using semiconductors that exhibit the photovoltaic effect. Photovoltaic power generation employs solar panels composed of a number of solar cells containing a photovoltaic material... |
106 | ||
| Wind power Wind power Wind power is the conversion of wind energy into a useful form of energy, such as using wind turbines to make electricity, windmills for mechanical power, windpumps for water pumping or drainage, or sails to propel ships.... |
21 |
Legend: B = Black coal (supercritical)–(new subcritical), Br = Brown coal (new subcritical), cc = combined cycle, oc = open cycle, TL = low-temperature/closed-circuit (geothermal doublet), TH = high-temperature/open-circuit, WL = Light Water Reactors, WH = Heavy Water Reactors, #Educated estimate.
World CO2 intensity in 2009
In 2009 CO2 intensity in the OECD countries reduced by 2.9% and amounted to 0.33 kCO2/$05p in the OECD countries.
The USA posted a higher ratio of 0.41 kCO2/$05p while Europe showed the largest drop in CO2 intensity compared to the previous year (-3.7%). CO2 intensity continued to be roughly higher in non-OECD countries. Despite a slight improvement, China continued to post a high CO2 intensity (0.81 kCO2/$05p). CO2 intensity in Asia rose by 2% during 2009 since energy consumption continued to develop at a strong pace. Important ratios were also observed in countries in CIS and the Middle East.
Carbon intensity in Europe
Total CO2 emissions from energy use were 5 % below their 1990 level in 2007. Over the period 1990-2007, CO2 emissions from energy use have decreased on average by 0.3 %/year although the economic activity (GDP) increased by 2.3 %/year. After dropping until 1994 (-1.6 %/year), the CO2 emissions have increased steadily (0.4 %/year on average) until 2003 and decreased slowly again since (on average by 0.6 %/year). Total CO2 emissions per capita decreased from 8.7 t in 1990 to 7.8 t in 2007, that is to say a decrease by 10 %.
Almost 40 % of the reduction in CO2 intensity is due to increased use of energy carriers with lower emission factors
Total CO2 emissions per unit of GDP, the “CO2 intensity”, decreased more rapidly than energy intensity: by 2.3 %/year and 1.4 %/year, respectively, on average between 1990 and 2007.
Emission factors for greenhouse gas inventory reporting
One of the most important uses of emission factors is for the reporting of national greenhouse gas inventories
Greenhouse gas inventory
Greenhouse gas inventories are a type of emission inventory that are developed for a variety of reasons. Scientists use inventories of natural and anthropogenic emissions as tools when developing atmospheric models. Policy makers use inventories to develop strategies and policies for emissions...
under the United Nations Framework Convention on Climate Change
United Nations Framework Convention on Climate Change
The United Nations Framework Convention on Climate Change is an international environmental treaty produced at the United Nations Conference on Environment and Development , informally known as the Earth Summit, held in Rio de Janeiro from June 3 to 14, 1992...
(UNFCCC). The so-called Annex I Parties to the UNFCCC have to annually report their national total emissions of greenhouse gases in a formalized reporting format, defining the source categories and fuels that must be included.
UNFCCC has accepted the Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventorieshttp://www.ipcc-nggip.iges.or.jp/public/gl/invs6.htm, developed and published by the Intergovernmental Panel on Climate Change
Intergovernmental Panel on Climate Change
The Intergovernmental Panel on Climate Change is a scientific intergovernmental body which provides comprehensive assessments of current scientific, technical and socio-economic information worldwide about the risk of climate change caused by human activity, its potential environmental and...
(IPCC) as the emission estimation methods that must be used by the parties to the convention to ensure transparency, completeness, consistency, comparability and accuracy of the national greenhouse gas inventories http://unfccc.int/resource/docs/2004/sbsta/08.pdf. These IPCC Guidelines are the primary source for default emission factors. Recently IPCC has published the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. These and many more greenhouse gas emission factors can be found on IPCC's Emission Factor Database http://www.ipcc-nggip.iges.or.jp/EFDB/main.php.
Particularly for non-CO2 emissions, there is often a high degree of uncertainty associated with these emission factors when applied to individual countries. In general, the use of country-specific emission factors would provide more accurate estimates of emissions than the use of the default emission factors. According to the IPCC, if an activity is a major source of emissions for a country ('key source'), it is 'good practice' to develop a country-specific emission factor for that activity.
Emission factors for air pollutant inventory reporting
National Air Pollution Emission Inventories are required annually under the provisions of the UNECE Convention on Long-Range Transboundary Air Pollution
Convention on Long-Range Transboundary Air Pollution
The Convention on Long-Range Transboundary Air Pollution, often abbreviated as Air Pollution or CLRTAP, is intended to protect the human environment against air pollution and to gradually reduce and prevent air pollution, including long-range transboundary air pollution.-Overview:The convention...
(LRTAP). Emission estimation methods and the associated emission factors for air pollutants have been developed by the EMEP Task Force on Emission Inventories and Projections (TFEIP) and are published in the EMEP/CORINAIR Emission Inventory Guidebook.
Intensity targets

From 1990 to 2000, the carbon intensity of the U.S. economy declined by 17%, yet total emissions increased by 14%. In 2002, the U.S. National Environmental trust labelled carbon intensity, "a bookkeeping trick which allows the administration to do nothing about global warming while unsafe levels of emissions continue to rise."
Greenhouse gases
- 2006 IPCC Guidelines for National Greenhouse Gas Inventories
- Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventories (reference manual).
- IPCC Emission Factor Database
- National Inventory Report: Greenhouse Gas Sources and Sinks in Canada.
- United Kingdom's emission factor database.
Air pollutants
- AP 42, Compilation of Air Pollutant Emission Factors US Environmental Protection Agency
- EMEP/CORIMAIR 2007 Emission Inventory Guidebook.
- Fugitive emissions leaks from ethylene and other chemical plants.
See also
- Energy intensityEnergy intensity]Energy intensity is a measure of the energy efficiency of a nation's economy. It is calculated as units of energy per unit of GDP.* High energy intensities indicate a high price or cost of converting energy into GDP....
- Carbon footprintCarbon footprintA carbon footprint has historically been defined as "the total set of greenhouse gas emissions caused by an organization, event, product or person.". However, calculating a carbon footprint which conforms to this definition is often impracticable due to the large amount of data required, which is...
- List of countries by ratio of GDP to carbon dioxide emissions
- Low carbon economy
- Low-carbon fuel standardLow-carbon fuel standardA low-carbon fuel standard is a rule enacted to reduce carbon intensity in transportation fuels as compared to conventional petroleum fuels, such as gasoline and diesel. The most common low-carbon fuels are alternative fuels and cleaner fossil fuels, such as natural gas...
- Emission inventoryEmission inventoryAn emission inventory is an accounting of the amount of pollutants discharged into the atmosphere. An emission inventory usually contains the total emissions for one or more specific greenhouse gases or air pollutants, originating from all source categories in a certain geographical area and within...
- Air pollutionAir pollutionAir pollution is the introduction of chemicals, particulate matter, or biological materials that cause harm or discomfort to humans or other living organisms, or cause damage to the natural environment or built environment, into the atmosphere....
- AP 42 Compilation of Air Pollutant Emission FactorsAP 42 Compilation of Air Pollutant Emission FactorsThe AP 42 Compilation of Air Pollutant Emission Factors, was first published by the U.S. Public Health Service in 1968. In 1972, it was revised and issued as the second edition by the U.S. Environmental Protection Agency . In 1985, the subsequent fourth edition was split into two volumes...
- Emission standardEmission standardEmission standards are requirements that set specific limits to the amount of pollutants that can be released into the environment. Many emissions standards focus on regulating pollutants released by automobiles and other powered vehicles but they can also regulate emissions from industry, power...
- Greenhouse gasGreenhouse gasA greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
and Greenhouse effectGreenhouse effectThe greenhouse effect is a process by which thermal radiation from a planetary surface is absorbed by atmospheric greenhouse gases, and is re-radiated in all directions. Since part of this re-radiation is back towards the surface, energy is transferred to the surface and the lower atmosphere... - IPCC list of greenhouse gasesIPCC list of greenhouse gasesThis is a list of LLGHG greenhouse gases as used by the IPCC TAR.-Gases relevant to radiative forcing only :The following table has its sources in Chapter 2, pg 141, Table 2.1...
- Mobile Emission Reduction Credit (MERC)Mobile Emission Reduction Credit (MERC)A mobile emission reduction credit is an emission reduction credit generated within the transportation sector. The term “mobile sources” refers to motor vehicles, engines, and equipment that move, or can be moved, from place to place...
- Radiative forcingRadiative forcingIn climate science, radiative forcing is generally defined as the change in net irradiance between different layers of the atmosphere. Typically, radiative forcing is quantified at the tropopause in units of watts per square meter. A positive forcing tends to warm the system, while a negative...
- Kaya identityKaya identityThe Kaya identity is an equation relating factors that determine the level of human impact on climate, in the form of emissions of the greenhouse gas carbon dioxide. It states that total emission level can be expressed as the product of four inputs: population, GDP per capita, energy use per unit...
External links

