
Epothilone
Encyclopedia
| Epothilones | |
|---|---|
 |
|
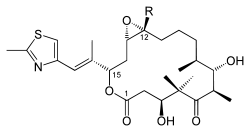
| Chemical formula Chemical formula A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound.... e |
A: C26H39NO6S B: C27H41NO6S |
| Molecular mass Molecular mass The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u... es |
A: 493.66 g/mol B: 507.68 g/mol |
| CAS numbers CAS registry number CAS Registry Numbersare unique numerical identifiers assigned by the "Chemical Abstracts Service" toevery chemical described in the... |
A: 152044-53-6 B: 152044-54-7 |
| PubChem PubChem PubChem is a database of chemical molecules and their activities against biological assays. The system is maintained by the National Center for Biotechnology Information , a component of the National Library of Medicine, which is part of the United States National Institutes of Health . PubChem can... |
A: 448799 B: 448013 |
 |
|
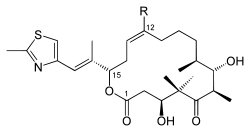
| Chemical formula Chemical formula A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound.... e |
C: C26H39NO5S D: C27H41NO5S |
| Molecular mass Molecular mass The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u... es |
C: 477.66 g/mol D: 491.68 g/mol |
| CAS numbers CAS registry number CAS Registry Numbersare unique numerical identifiers assigned by the "Chemical Abstracts Service" toevery chemical described in the... |
D: 189453-10-9 |
| PubChem PubChem PubChem is a database of chemical molecules and their activities against biological assays. The system is maintained by the National Center for Biotechnology Information , a component of the National Library of Medicine, which is part of the United States National Institutes of Health . PubChem can... |
C: 9891226 D: 447865 |
 |
|
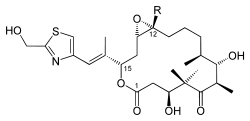
| Chemical formula Chemical formula A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound.... e |
E: C26H39NO7S F: C27H41NO7S |
| Molecular mass Molecular mass The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u... es |
E: 509.66 g/mol F: 523.68 g/mol |
| Disclaimer and references | |
The epothilones are a new class of cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin
Tubulin
Tubulin is one of several members of a small family of globular proteins. The most common members of the tubulin family are α-tubulin and β-tubulin, the proteins that make up microtubules. Each has a molecular weight of approximately 55 kiloDaltons. Microtubules are assembled from dimers of α- and...
, but in early trials epithilones have better efficacy and milder adverse effects than taxanes.
, epothilones A to F have been identified and characterised.
Early studies in cancer cell lines and in human cancer patients indicate superior efficacy to the taxane
Taxane
The taxanes are diterpenes produced by the plants of the genus Taxus . As their name suggests, they were first derived from natural sources, but some have been synthesized artificially. Taxanes include paclitaxel and docetaxel . Paclitaxel was originally derived from the Pacific yew tree.Taxanes...
s. Their mechanism of action is similar, but their chemical structure is simpler. Due to their better water solubility, cremophors (solubilizing agents used for paclitaxel
Paclitaxel
Paclitaxel is a mitotic inhibitor used in cancer chemotherapy. It was discovered in a U.S. National Cancer Institute program at the Research Triangle Institute in 1967 when Monroe E. Wall and Mansukh C. Wani isolated it from the bark of the Pacific yew tree, Taxus brevifolia and named it taxol...
which can affect cardiac function and cause severe hypersensitivity) are not needed.
Endotoxin-like properties known from paclitaxel, like activation of macrophages synthesizing inflammatory cytokines and nitric oxide, are not observed for epothilone B.
Epothilones were originally identified as metabolites produced by the soil-dwelling myxobacterium Sorangium cellulosum
Sorangium cellulosum
Sorangium cellulosum is a soil-dwelling Gram-negative bacterium of the group myxobacteria. It is motile and shows gliding motility. It has an unusually-large genome of 13,033,779 base pairs, making it the largest bacterial genome sequenced to date....
.
History
The structure of epothilone A was determined in 1996 using x-ray crystallographyX-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
.
Mechanism of action
The principal mechanism of the epothilone class is inhibition of microtubuleMicrotubule
Microtubules are a component of the cytoskeleton. These rope-like polymers of tubulin can grow as long as 25 micrometers and are highly dynamic. The outer diameter of microtubule is about 25 nm. Microtubules are important for maintaining cell structure, providing platforms for intracellular...
function. Microtubules are essential to cell division, and epothilones therefore stop cells from properly dividing. Epothilone B possess the same biological effects as paclitaxel both in vitro and in cultured cells. This is because they share the same binding site, as well as binding affinity to the microtubule. Like paclitaxel, epothilone B binds to the αβ-tubulin heterodimer subunit. Once bound, the rate of αβ-tubulin dissociation decreases, thus stabilizing the microtubules. Furthermore, epothilone B has also been shown to induce tubulin polymerization into microtubules without the presence of GTP. This is caused by formation of microtubule bundles throughout the cytoplasm. Finally, epothilone B also causes cell cycle arrest at the G2-M transition phase, thus leading to cytotoxicity and eventually cell apoptosis. The ability of epothilone to inhibit spindle function is generally attributed to its suppression of microtubule dynamics; but recent studies have demonstrated that suppression of dynamics occurs at concentrations lower than those needed to block mitosis. At the higher antimitotic concentrations, paclitaxel appears to act by suppressing microtubule detachment from centrosomes, a process that is normally activated during mitosis. It is quite possible that epothilone can also act though similar mechanism.
Clinical trials
Several epothilone analogs are currently undergoing clinical development for treatment of various cancers. One analog, ixabepiloneIxabepilone
Ixabepilone is an epothilone B analog developed by Bristol-Myers Squibb as a cancer drug.It is produced by Sorangium cellulosum.-Pharmacology:It acts to stabilize microtubules....
, was approved in October 2007 by the United States Food and Drug Administration
Food and Drug Administration
The Food and Drug Administration is an agency of the United States Department of Health and Human Services, one of the United States federal executive departments...
for use in the treatment of aggressive metastatic or locally advanced breast cancer no longer responding to currently available chemotherapies. In November 2008, the EMEA
European Medicines Agency
The European Medicines Agency is a European agency for the evaluation of medicinal products. From 1995 to 2004, the European Medicines Agency was known as European Agency for the Evaluation of Medicinal Products.Roughly parallel to the U.S...
has refused a marketing authorisation for Ixabepilone.
Epothilone B has proven to contain potent in vivo anticancer activities at tolerate dose levels in several human xenograft models. As a result, epothilone B and its various analogues are currently undergoing various clinical phases (patupilone [EPO906] and sagopilone [SH-Y03757A, ZK-EPO, chemical structure] are in phase II trials; BMS-310705 and BMS-247550 in phase I trials). Results of a phase III trial with ixabepilone
Ixabepilone
Ixabepilone is an epothilone B analog developed by Bristol-Myers Squibb as a cancer drug.It is produced by Sorangium cellulosum.-Pharmacology:It acts to stabilize microtubules....
in combination with capecitabine in metastatic breast cancer have been announced.
Patupilone failed a phase III trial for ovarian cancer
Ovarian cancer
Ovarian cancer is a cancerous growth arising from the ovary. Symptoms are frequently very subtle early on and may include: bloating, pelvic pain, difficulty eating and frequent urination, and are easily confused with other illnesses....
.
Total synthesis
Due to the high potency and clinical need for cancer treatments, epothilones have been the target of many total synthesesTotal synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
. The first group to publish the total synthesis of epothilones was S. J. Danishefsky et al. in 1996. This total synthesis of epothilone A was achieved via an intramolecular ester enolate-aldehyde condensation. Other syntheses of epothilones have been published by Nicolaou
Kyriacos Costa Nicolaou
Kyriacos Costa Nicolaou is a Cypriot-American chemist known for the total synthesis of natural products.-Biography:K. C. Nicolaou was born on July 5, 1946, in Karavas, Cyprus where he grew up and went to school until the age of 18. In 1964, he went to England where he spent two years learning...
, Schinzer, Mulzer, and Carreira. In this approach, key building blocks aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
, glycidol
Glycidol
Glycidol is an organic compound that contains both epoxide and alcohol functional groups. Being bifunctional, it has a variety of industrial uses...
s, and ketoacid were constructed and coupled to olefin metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
precursor via an aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
and then an esterification coupling. Grubbs' catalyst
Grubbs' catalyst
Grubbs' Catalyst is a transition metal carbene complex named after Robert H. Grubbs, the chemist who first synthesized it. There are two generations of the catalyst, as shown on the right. In contrast to other olefin metathesis catalysts, Grubbs' Catalysts tolerate other functional groups in the...
was employed to close the bis terminal olefin of the precursor compound. The resulting compounds were cis- and tran-macrocyclic isomers with distinct stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
s. Epoxidation of cis- and trans-olefins yield epothilone A and its analogues.
The particular synthetic method determined by the laboratories of K.C Nicolaou, described the synthesis of appropriate building blocks 9, 11, and 12, derived from the retrosynthetic analysis of epothilone B (Figure 1), both diastereoisomers and the geometrical isomers at C6-C7 and C12-C13, can be obtained to give a diverse molecular product. The synthesis of required building blocks 9, 11 and 12, were obtained in a maximum of 4 steps for each building block as seen in Figure 2. With fragments 9, 11 and 12 in hand, these intermediates can then react with one another via Wittig olefination, aldol reaction, macrolactonization, and epoxidation to give the various epothilone B as seen in Figure 3.
Biosynthesis
Epothilone B is a 16-membered polyketidePolyketide
Polyketides are secondary metabolites from bacteria, fungi, plants, and animals. Polyketides are usually biosynthesized through the decarboxylative condensation of malonyl-CoA derived extender units in a similar process to fatty acid synthesis...
macrolactone with a methylthiazole
Thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS...
group connected to the macrocycle by an olefinic bond. The polyketide backbone was synthesized by type I polyketide synthase
Polyketide synthase
Polyketide synthases are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages...
(PKS) and the thiazole ring was derived from a cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
incorporated by a nonribosomal peptide synthetase (NRPS). In this biosythesis, both PKS and NRPS use carrier protein
Carrier protein
Carrier proteins are proteins involved in the movement of ions, small molecules, or macromolecules, such as another protein, across a biological membrane. Carrier proteins are integral membrane proteins; that is they exist within and span the membrane across which they transport substances. The...
s, which have been post-translationally modified by phosphopantheteine groups, to join the growing chain. PKS uses coenzyme-A thioester to catalyze the reaction and modify the substrates by selectively reducing the β carbonyl to the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
(Ketoreductase, KR), the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
(Dehydratase, DH), and the alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
(Enoyl Reductase, ER). PKS-I can also methylate
Methylation
In the chemical sciences, methylation denotes the addition of a methyl group to a substrate or the substitution of an atom or group by a methyl group. Methylation is a form of alkylation with, to be specific, a methyl group, rather than a larger carbon chain, replacing a hydrogen atom...
the α carbon of the substrate. NRPS, on the other hand, uses amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s activated on the enzyme as aminoacyl adenylates. Unlike PKS, epimerization, N-methylation, and heterocycle formation occurs in NRPS enzyme.
Epothilone B starts with a 2-methyl-4-carboxythiazole starter unit, which was formed through the translational coupling between PKS, EPOS A (epoA) module, and NRPS, EPOS P(epoP) module. The EPOS A contains a modified β-ketoacyl-synthase (malonyl-ACP decarboxylase, KSQ), an acyltransferase (AT), an enoyl reductase (ER), and an acyl carrier protein domain (ACP). The EPOS P however, contains a heterocylization, an adenylation, an oxidase, and a thiolation domain. These domains are important because they are involved in the formation of the five-membered heterocyclic ring of the thiazole. As seen in Figure 4, the EPOS P activates the cysteine and binds the activated cysteine as an aminoacyl-S-PCP. Once the cysteine has been bound, EPOS A loads an acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
unit onto the EPOS P complex, thus initiating the formation of the thiazoline ring by intramolecular cyclodehydration.
Once the 2-methylthiazole ring has been made, it is then transferred to the PKS EPOS B (epoB), EPOS C (epoC), EPOS D (epoD), EPOS E (epoE), and EPOS F (epoF) for subsequent elongation and modification to generate the olefinic bond, the 16-membered ring, and the epoxide, as seen in Figure 5. One important thing to note is the synthesis of the gem-dimethyl unit in module 7. These two dimethyls were not synthesized by two successive C-methylations. Instead one of the methyl group
Methyl group
Methyl group is a functional group derived from methane, containing one carbon atom bonded to three hydrogen atoms —CH3. The group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. The methyl group can be found in three forms: anion, cation and radical. The anion...
was derived from the propionate extender unit, while the second methyl group was integrated by a C-methyl-transferase domain.

