Thiazole
Encyclopedia
Thiazole, or 1,3-thiazole, is a heterocyclic compound
that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine
-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin
thiamine
(B1).
s heterocycles that includes imidazoles and oxazoles. Thiazole can also be considered a functional group
. Oxazole
s are related compounds, with sulfur replaced by oxygen. Thiazoles are structurally similar to imidazole
s, with the thiazole sulfur replaced by nitrogen.
Thiazole rings are planar and aromatic
Thiazoles are characterized by larger pi-electron delocalization than the corresponding oxazole
s and have therefore greater aromaticity
. This aromaticity is evidenced by the chemical shift of the ring protons in proton NMR
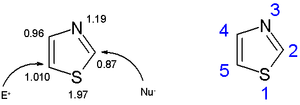
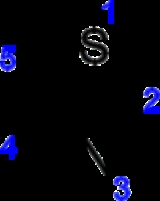
spectroscopy (between 7.27 and 8.77 ppm), clearly indicating a strong diamagnetic ring current. The calculated pi-electron density marks C5 as the primary site for electrophilic substitution, and C2 as the site for nucleophilic substitution.
. Other important thiazole dervatives are benzothiazole
s, for example, the firefly chemical luciferin
. Whereas thiazoles are well represented in biomolecule
s, oxazoles are not.
Commercial significant thiazoles include mainly dyes and fungicide
s. Thifluzamide, Tricyclazole, and Thiabendazole
are marketed for control of various agricultural pests. Another widely used thiazole derivative is the non-steroidal anti-inflammatory drug Meloxicam
. The following anthroquinone dyes contain benzothiazole subunits: Algol Yellow 8 (CAS# [6451-12-3]), Algol Yellow GC (CAS# [129-09-9]), Indanthren Rubine B (CAS# [6371-49-9]), Indanthren Blue CLG (CAS# [6371-50-2], and Indanthren Blue CLB (CAS#[6492-78-0]). These thiazole dye are used for dying cotton
.
of thiazoles.
of thiazoles at nitrogen forms a thiazolium cation. Thiazolium salts are catalysts in the Stetter reaction
and the Benzoin condensation
. Deprotonation of N-alkyl thiazolium salts give the free carbenes and transition metal carbene complex
es.
Alagebrium
is a thiazolium-based drug.
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin
Vitamin
A vitamin is an organic compound required as a nutrient in tiny amounts by an organism. In other words, an organic chemical compound is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on...
thiamine
Thiamine
Thiamine or thiamin or vitamin B1 , named as the "thio-vitamine" is a water-soluble vitamin of the B complex. First named aneurin for the detrimental neurological effects if not present in the diet, it was eventually assigned the generic descriptor name vitamin B1. Its phosphate derivatives are...
(B1).
Molecular and electronic structure
Thiazoles are members of the azoleAzole
An azole is a class of five-membered nitrogen heterocyclic ring compounds containing at least one other non-carbon atom of either nitrogen, sulfur, or oxygen. The parent compounds are aromatic and have two double bonds; there are successively reduced analogs with fewer...
s heterocycles that includes imidazoles and oxazoles. Thiazole can also be considered a functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
. Oxazole
Oxazole
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles...
s are related compounds, with sulfur replaced by oxygen. Thiazoles are structurally similar to imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
s, with the thiazole sulfur replaced by nitrogen.
Thiazole rings are planar and aromatic
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
Thiazoles are characterized by larger pi-electron delocalization than the corresponding oxazole
Oxazole
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles...
s and have therefore greater aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
. This aromaticity is evidenced by the chemical shift of the ring protons in proton NMR
Proton NMR
Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen is used, practically all of the hydrogen consists of the...
spectroscopy (between 7.27 and 8.77 ppm), clearly indicating a strong diamagnetic ring current. The calculated pi-electron density marks C5 as the primary site for electrophilic substitution, and C2 as the site for nucleophilic substitution.
Occurrence of thiazoles and thiazolium salts
Thiazoles are found is a variety of specialized products, often fused with benzene derivatives, the so-called benzothiazoles. In addition to vitamin B1, the thiazole ring is found in epothiloneEpothilone
The epothilones are a new class of cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin, but in early trials epithilones have better efficacy and milder adverse effects than taxanes....
. Other important thiazole dervatives are benzothiazole
Benzothiazole
Benzothiazole is an aromatic heterocyclic compound with the chemical formula . It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature...
s, for example, the firefly chemical luciferin
Luciferin
Luciferins are a class of light-emitting biological pigments found in organisms that cause bioluminescence...
. Whereas thiazoles are well represented in biomolecule
Biomolecule
A biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
s, oxazoles are not.
Commercial significant thiazoles include mainly dyes and fungicide
Fungicide
Fungicides are chemical compounds or biological organisms used to kill or inhibit fungi or fungal spores. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality and profit. Fungicides are used both in agriculture and to fight fungal infections in animals...
s. Thifluzamide, Tricyclazole, and Thiabendazole
Thiabendazole
Tiabendazole is a fungicide and parasiticide.-Fungicide:...
are marketed for control of various agricultural pests. Another widely used thiazole derivative is the non-steroidal anti-inflammatory drug Meloxicam
Meloxicam
Meloxicam is a nonsteroidal anti-inflammatory drug with analgesic and fever reducer effects. It is a derivative of oxicam, closely related to piroxicam, and falls in the enolic acid group of NSAIDs...
. The following anthroquinone dyes contain benzothiazole subunits: Algol Yellow 8 (CAS# [6451-12-3]), Algol Yellow GC (CAS# [129-09-9]), Indanthren Rubine B (CAS# [6371-49-9]), Indanthren Blue CLG (CAS# [6371-50-2], and Indanthren Blue CLB (CAS#[6492-78-0]). These thiazole dye are used for dying cotton
Cotton
Cotton is a soft, fluffy staple fiber that grows in a boll, or protective capsule, around the seeds of cotton plants of the genus Gossypium. The fiber is almost pure cellulose. The botanical purpose of cotton fiber is to aid in seed dispersal....
.
Organic synthesis
Various laboratory methods exist for the organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of thiazoles.
- The Hantzsch thiazole synthesis (1889) is a reaction between haloketoneHaloketoneA haloketone in organic chemistry is a functional group consisting of a ketone group or more general a carbonyl group with a α-halogen substituent. The general structure is RR'CCR where R is an alkyl or aryl residue and X any one of the halogens...
s and thioamideThioamideThioamide is a functional group with the general structure R-CS-NR'R, where R, R', and R are organic groups. They are analogous to amides but they exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier...
s. For example, 2,4-dimethylthiazole is synthesized from acetamideAcetamideAcetamide is an organic compound with the formula CH3CONH2. It is the simplest amide derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent...
, phosphorus pentasulfidePhosphorus pentasulfidePhosphorus pentasulfide is the inorganic compound with the formula P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value...
, and chloroacetoneChloroacetoneChloroacetone is a chemical compound with the formula 3CCH2. At STP it is a colourless liquid with a pungent odour. On exposure to light, it turns to a dark yellow-amber colour. It was used as a tear gas in World War I.-Applications:...
. Another example is given below:
- In an adaptation of the Robinson-Gabriel synthesisRobinson-Gabriel synthesisThe Robinson-Gabriel synthesis is a chemical reaction that forms oxazoles by dehydration of 2-acylamino-ketones.Historically, the dehydration agent is concentrated sulfuric acid. Recently, phosphorus oxychloride is successful with this reaction also....
, a 2-acylamino-ketones reacts with phosphorus pentasulfidePhosphorus pentasulfidePhosphorus pentasulfide is the inorganic compound with the formula P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value...
. - In the Cook-Heilbron synthesis, an α-aminonitrile reacts with carbon disulfideCarbon disulfideCarbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
. - Certain thiazoles can be accessed though application of the Herz reactionHerz reactionThe Herz-reaction, named after the chemist Richard Herz, is the chemical conversion of an aniline-derivative to a so-called Herz-salt with disulfur dichloride, followed by hydrolysis of this Herz-salt to the corresponding sodium thiolate :-Benzothiazoles:The sodium thiolate 3 can be converted to...
.
Biosynthesis
Several biosynthesis routes lead to the thiazole ring as required for the formation of thiamine. Sulfur of the thiazole is derived from cysteine. In anaerobic bacteria, the CN group is derived from dehydroglycine.Reactions
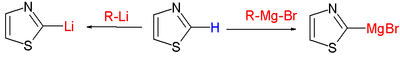
The reactivity of a thiazole can be summarized as follows:- DeprotonationDeprotonationDeprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
at C2: the negative charge on this position is stabilized as an ylideYlideAn ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
; Grignard reagents and organolithium compounds react at this site, replacing the proton
- 2-(trimethylsiliyl)thiazole (with a trimethylsilylTrimethylsilylA trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
group in the 2-position) is a stable substitute and reacts with a range of electrophiles such as aldehydeAldehydeAn aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s, acyl halideAcyl halideAn acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group....
s, and keteneKeteneA ketene is an organic compound of the form R'RC=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R are hydrogen atoms.Ketenes were first studied as a class by Hermann Staudinger.-Formation:...
s
- Electrophilic aromatic substitutionElectrophilic aromatic substitutionElectrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
at C5 requires activating groupActivating groupIn organic chemistry, a functional group is called an activating group if a benzene molecule to which it is attached more readily participates in electrophilic substitution reactions...
s such as a methyl group in this bromination:
- Nucleophilic aromatic substitutionNucleophilic aromatic substitutionright|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring...
often requires an electrofugeElectrofugeAn electrofuge is a leaving group which does not retain the bonding pair of electrons from its previous bond with another species.After this reaction an electrofuge may possess either a positive or a neutral charge; this is governed by the nature of the specific reaction.An example would be the...
at C2, such as chlorineChlorineChlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
with
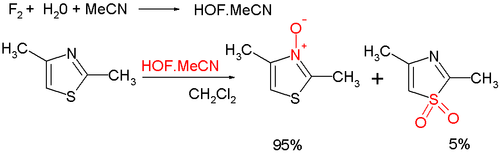
- Organic oxidation at nitrogen gives the thiazole N-oxide; many oxidizing agents exist, such as mCPBA; a novel one is hypofluorous acid prepared from fluorineFluorineFluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
and water in acetonitrileAcetonitrileAcetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
; some of the oxidation takes place at sulfur, leading to a sulfoxideSulfoxideA sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
:
- Thiazoles are formyl synthonSynthonA synthon is a concept in retrosynthetic analysis. It is defined as a structural unit within a molecule which is related to a possible synthetic operation. The term was coined by E.J. Corey...
s; conversion of R-thia to the R-CHO aldehyde takes place with, respectively, methyl iodide (N-methylation), organic reduction with sodium borohydrideSodium borohydrideSodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
, and hydrolysisHydrolysisHydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
with Mercury(II) chlorideMercury(II) chlorideMercury chloride or mercuric chloride , is the chemical compound with the formula HgCl2. This white crystalline solid is a laboratory reagent and a molecular compound. It is no longer used for medicinal purposes Mercury(II) chloride or mercuric chloride (formerly corrosive sublimate), is the...
in water.
- Thiazoles are formyl synthon
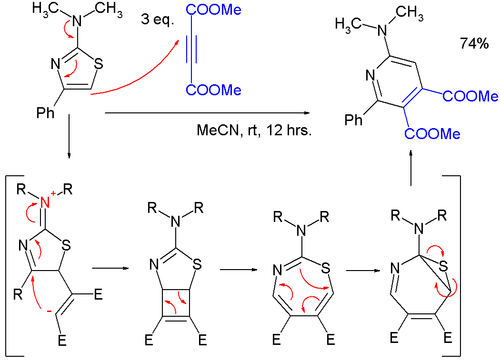
- Thiazoles can react in cycloadditionCycloadditionA cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
s, but in general at high temperatures due to favorable aromatic stabilization of the reactant; Diels-Alder reactionDiels-Alder reactionThe Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
s with alkyneAlkyneAlkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s are followed by extrusion of sulfur, and the endproduct is a pyridinePyridinePyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
; in one study, a very mild reaction of a 2-(dimethylamino)thiazole with dimethyl acetylenedicarboxylate (DMAD) to a pyridine was found to proceed through a zwitterionic intermediate in a formal [2+2]cycloaddition to a cyclobutene, then to a 1,3-thiazepine in an 4-electron electrocyclic ring openeningElectrocyclic reactionIn organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice-versa...
and then to a 7-thia-2-azanorcaradiene in an 6-electron electrocyclic ring, closing before extruding the sulfur atom.
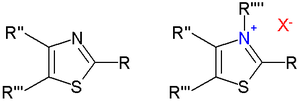
Thiazolium salts
AlkylationAlkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of thiazoles at nitrogen forms a thiazolium cation. Thiazolium salts are catalysts in the Stetter reaction
Stetter reaction
The Stetter reaction is an organic reaction involving the nucleophile catalyzed conjugate addition of an aldehyde to a Michael acceptor such as an enone. The reaction product is a 1,4-dicarbonyl compound...
and the Benzoin condensation
Benzoin condensation
The benzoin condensation is a reaction between two aromatic aldehydes, particularly benzaldehyde. The reaction is catalyzed by a nucleophile such as the cyanide anion or an N-heterocyclic carbene. The reaction product is an aromatic acyloin with benzoin as the parent compound...
. Deprotonation of N-alkyl thiazolium salts give the free carbenes and transition metal carbene complex
Transition metal carbene complex
A transition metal carbene complex is a organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been reported. Many methods for synthesizing them and...
es.
Alagebrium
Alagebrium
Alagebrium is a failed drug candidate developed by Alteon Corporation. It was the first drug to be clinically tested for the purpose of breaking the crosslinks caused by advanced glycation endproducts , thereby reversing one of the main mechanisms of aging...
is a thiazolium-based drug.