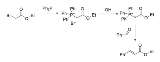
Ethyl bromoacetate
Encyclopedia
Ethyl 2-bromoacetate is the chemical compound
with the formula CH2BrCO2C2H5. It is the ethyl
ester
of bromoacetic acid
and is prepared in two steps from acetic acid
.
, ethyl bromoacetate was used as a lachrymatory agent
and tear gas agent for chemical warfare
under the German code Weisskreuz
(White Cross), and later as odorant or warning agent in odourless, toxic gases. It is listed by the WHO
as a riot control agent, and was first employed for that purpose by French police in 1912. The French may have employed gas grenades of this substance in 1914 during World War I
. The German army then used this attack to justify their subsequent employment of chemical weapons in 1915.
In organic synthesis
, it is a versatile alkylating agent. Its major application involves the Reformatsky reaction, wherein it reacts with zinc
to form a zinc enolate. The resulting BrZnCH2CO2Et condenses with carbonyl
compounds to give a β-hydroxy-esters.
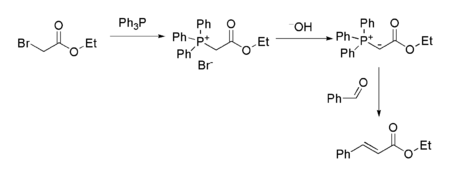
It is also the starting point for the preparation of several other reagents. For example, the related Wittig reagent (prepared by reaction with triphenylphosphine
) is commonly used to prepare alpha,beta-unsaturated
ester
s from carbonyl compounds such as benzaldehyde
:
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula CH2BrCO2C2H5. It is the ethyl
Ethyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
of bromoacetic acid
Bromoacetic acid
Bromoacetic acid is the chemical compound with the formula CH2BrCO2H. This colorless solid is a relatively strong alkylating agent. Bromoacetic acid and its esters are widely used building blocks in organic synthesis, for example in pharmaceutical chemistry....
and is prepared in two steps from acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
.
Applications
In World War IWorld War I
World War I , which was predominantly called the World War or the Great War from its occurrence until 1939, and the First World War or World War I thereafter, was a major war centred in Europe that began on 28 July 1914 and lasted until 11 November 1918...
, ethyl bromoacetate was used as a lachrymatory agent
Lachrymatory agent
Tear gas, formally known as a lachrymatory agent or lachrymator , is a non-lethal chemical weapon that stimulates the corneal nerves in the eyes to cause tears, pain, and even blindness...
and tear gas agent for chemical warfare
Chemical warfare
Chemical warfare involves using the toxic properties of chemical substances as weapons. This type of warfare is distinct from Nuclear warfare and Biological warfare, which together make up NBC, the military acronym for Nuclear, Biological, and Chemical...
under the German code Weisskreuz
White Cross (chemical warfare)
White Cross is a World War I chemical warfare agent consisting of one or more lachrymatory agents: bromoacetone , bromobenzyl cyanide , bromomethyl ethyl ketone , chloroacetone , ethyl bromoacetate, and/or xylyl bromide.During World war I, White Cross was also a generic code name used by the...
(White Cross), and later as odorant or warning agent in odourless, toxic gases. It is listed by the WHO
Who
Who may refer to:* Who , an English-language pronoun* who , a Unix command* Who?, one of the Five Ws in journalism- Art and entertainment :* Who? , a 1958 novel by Algis Budrys...
as a riot control agent, and was first employed for that purpose by French police in 1912. The French may have employed gas grenades of this substance in 1914 during World War I
World War I
World War I , which was predominantly called the World War or the Great War from its occurrence until 1939, and the First World War or World War I thereafter, was a major war centred in Europe that began on 28 July 1914 and lasted until 11 November 1918...
. The German army then used this attack to justify their subsequent employment of chemical weapons in 1915.
In organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, it is a versatile alkylating agent. Its major application involves the Reformatsky reaction, wherein it reacts with zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
to form a zinc enolate. The resulting BrZnCH2CO2Et condenses with carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds to give a β-hydroxy-esters.
It is also the starting point for the preparation of several other reagents. For example, the related Wittig reagent (prepared by reaction with triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
) is commonly used to prepare alpha,beta-unsaturated
Unsaturated compound
In organic chemistry, a saturated compound is a chemical compound that has of a chain of carbon atoms linked together by single bonds and has hydrogen atoms filling all of the other bonding orbitals of the carbon atoms. Alkanes are an example of saturated compounds...
ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s from carbonyl compounds such as benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
:


