
Eutectic system
Encyclopedia
A eutectic system is a mixture
of chemical compounds or elements that has a single chemical composition that solidifies at a lower temperature than any other composition. This composition is known as the eutectic composition and the temperature is known as the eutectic temperature. On a phase diagram
the intersection
of the eutectic temperature and the eutectic composition gives the eutectic point. Not all binary alloys have a eutectic point; for example, in the silver-gold system the melt temperature (liquidus) and freeze temperature (solidus
) both increase monotonically as the mix changes from pure silver to pure gold.
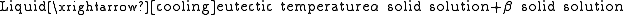
This type of reaction is an invariant reaction, because it is in thermal equilibrium
; another way to define this is the Gibbs free energy
equals zero. Tangibly, this means the liquid and two solid solution
s all coexist at the same time and are in chemical equilibrium
. There is also a thermal arrest for the duration of the reaction.
The resulting solid macrostructure
from a eutectic reaction depends on a few factors. The most important factor is how the two solid solutions nucleate and grow. The most common structure is a lamellar structure
, but other possible structures include rodlike, globular, and acicular.
s have two or more materials and have a eutectic composition. When a non-eutectic alloy solidifies, its components solidify at different temperatures, exhibiting a plastic melting range. A eutectic alloy solidifies at a single, sharp temperature. The phase transformations that occur while solidifying a given alloy can be understood by drawing a vertical line from the liquid phase to the solid phase on a phase diagram.
Some uses include:
and water
form a eutectic mixture. It has a eutectic point of −21.2 C and 23.3% salt by mass. The eutectic nature of salt and water is exploited when salt is spread on roads to aid snow removal
, or mixed with ice to produce low temperatures (for example, in traditional ice cream
making).
60% NaNO3 and 40% KNO3 forms a eutectic mixture which is used in solar molten salt technology.. To reduce the eutectic melting point in the solar molten salts the Calcium nitrate
is used in this proportion, 42% Ca(NO3)2, 43% KNO3 and 15% NaNO3.
Lidocaine
and prilocaine
, both solids at room temperature, form a eutectic that is an oil with a 16 °C (60.8 °F) melting point, used in eutectic mixture of local anesthetic (EMLA) preparations.
Mineral
s may form eutectic mixtures in igneous rocks, giving rise to characteristic intergrowth textures such as that of granophyre
.
Some inks are eutectic mixtures, allowing inkjet printer
s to operate at lower temperatures.
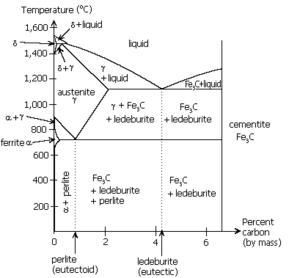 When the solution above the transformation point is solid, rather than liquid, an analogous eutectoid transformation can occur. For instance, in the iron-carbon system, the austenite phase can undergo a eutectoid transformation to produce ferrite
When the solution above the transformation point is solid, rather than liquid, an analogous eutectoid transformation can occur. For instance, in the iron-carbon system, the austenite phase can undergo a eutectoid transformation to produce ferrite
and cementite, often in lamellar structures such as pearlite
and bainite
. This eutectoid point occurs at 723 °C (1,333.4 °F) and about 0.83% carbon.
that have two solid phase
s reacting with each other upon cooling of a binary, ternary, ... , alloy
alloy
to create a completely different and single solid phase. The reaction plays a key role in the order and decomposition
of quasicrystalline phases in several alloy types.
Such a transformation exists in the iron-carbon system, as seen near the upper-left corner of the figure. It resembles an inverted eutectic, with the δ phase combining with the liquid to produce pure austenite at 1495 °C (2,723 °F) and 0.17% carbon.
Mixture
In chemistry, a mixture is a material system made up by two or more different substances which are mixed together but are not combined chemically...
of chemical compounds or elements that has a single chemical composition that solidifies at a lower temperature than any other composition. This composition is known as the eutectic composition and the temperature is known as the eutectic temperature. On a phase diagram
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
the intersection
Intersection
Intersection has various meanings in different contexts:*In mathematics and geometry**Intersection , the set of elements common to some collection of sets.**Line-line intersection**Line-plane intersection**Line–sphere intersection...
of the eutectic temperature and the eutectic composition gives the eutectic point. Not all binary alloys have a eutectic point; for example, in the silver-gold system the melt temperature (liquidus) and freeze temperature (solidus
Solidus
Solidus may refer to:*Solidus , the "⁄" grammatical punctuation character, also used in mathematics*Slash a sign, "/" used as a punctuation mark and for various other purposes...
) both increase monotonically as the mix changes from pure silver to pure gold.
Eutectic reaction
The eutectic reaction is defined as follows: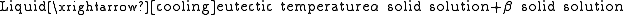
This type of reaction is an invariant reaction, because it is in thermal equilibrium
Thermal equilibrium
Thermal equilibrium is a theoretical physical concept, used especially in theoretical texts, that means that all temperatures of interest are unchanging in time and uniform in space...
; another way to define this is the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
equals zero. Tangibly, this means the liquid and two solid solution
Solid solution
A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase...
s all coexist at the same time and are in chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
. There is also a thermal arrest for the duration of the reaction.
The resulting solid macrostructure
Macrostructure
Macrostructure may refer to:*Macrostructure *Macrostructure *Macrostructure...
from a eutectic reaction depends on a few factors. The most important factor is how the two solid solutions nucleate and grow. The most common structure is a lamellar structure
Lamellar structure
Lamellar structures or microstructures are composed of fine, alternating layers of different materials in the form of lamellae. They are often observed in cases where a phase transformation front moves quickly, leaving behind two solid products, as in rapid cooling of eutectic or eutectoid ...
, but other possible structures include rodlike, globular, and acicular.
Non-eutectic compositions
Compositions of eutectic systems that are not the eutectic composition are commonly defined to be hypoeutectic or hypereutectic. Hypoeutectic composition are composition to the left of the eutectic composition and hypereutectic composition are compositions to the right.Alloys
Eutectic alloyAlloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
s have two or more materials and have a eutectic composition. When a non-eutectic alloy solidifies, its components solidify at different temperatures, exhibiting a plastic melting range. A eutectic alloy solidifies at a single, sharp temperature. The phase transformations that occur while solidifying a given alloy can be understood by drawing a vertical line from the liquid phase to the solid phase on a phase diagram.
Some uses include:
- eutectic alloys for solderingSolderingSoldering is a process in which two or more metal items are joined together by melting and flowing a filler metal into the joint, the filler metal having a lower melting point than the workpiece...
, composed of tinTinTin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
(Sn), leadLeadLead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
(Pb) and sometimes silverSilverSilver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
(Ag) or goldGoldGold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
(Au) - casting alloys, such as aluminiumAluminiumAluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
-siliconSiliconSilicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
and cast ironCast ironCast iron is derived from pig iron, and while it usually refers to gray iron, it also identifies a large group of ferrous alloys which solidify with a eutectic. The color of a fractured surface can be used to identify an alloy. White cast iron is named after its white surface when fractured, due...
(at the composition for an austeniteAusteniteAustenite, also known as gamma phase iron, is a metallic non-magnetic allotrope of iron or a solid solution of iron, with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of ; other alloys of steel have different eutectoid temperatures...
-cementiteCementiteCementite, also known as iron carbide, is a chemical compound of iron and carbon, with the formula Fe3C . By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, brittle material, normally classified as a ceramic in its pure form, though it is more...
eutectic in the ironIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
-carbonCarbonCarbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
system) - one method used by the semiconductorSemiconductorA semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
industry to bond silicon chipSilicon ChipSilicon Chip is an Australian electronics magazine. It was started in November, 1987 by Leo Simpson. Following the demise of Electronics Australia, it is the only hobbyist-related electronics magazine remaining in Australia.- Magazine :...
s to gold-plated substrates is to induce a silicon-gold eutectic through the application of ultrasonic energy to the chip - brazingBrazingBrazing is a metal-joining process whereby a filler metal is heated above and distributed between two or more close-fitting parts by capillary action. The filler metal is brought slightly above its melting temperature while protected by a suitable atmosphere, usually a flux...
, where diffusion can remove alloying elements from the joint, so that eutectic melting is only possible early in the brazing process - temperature response, i.e. Wood's metalWood's metalWood's metal, also known as Lipowitz's alloy or by the commercial names cerrobend, bendalloy, pewtalloy or MCP 158, is a eutectic, fusible alloy with a melting point of approximately . It is a eutectic alloy of 50% bismuth, 26.7% lead, 13.3% tin, and 10% cadmium by weight. It...
and Field's metalField's metalField's metal, or Field's alloy is a fusible alloy that becomes liquid at approximately . It is a eutectic alloy of bismuth, indium, and tin, with the following percentages by weight: 32.5% Bi, 51% In, 16.5% Sn....
for fire sprinklerFire sprinklerA fire sprinkler system is an active fire protection measure, consisting of a water supply system, providing adequate pressure and flowrate to a water distribution piping system, onto which fire sprinklers are connected...
s - non-toxic mercuryMercury (element)Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
replacements, such as galinstanGalinstanGalinstan is a family of eutectic alloys mainly consisting of gallium, indium, and tin, which are liquids at room temperature, typically freezing at . Due to the low toxicity and low reactivity of its component metals, it finds use as a replacement for many applications that previously employed... - experimental glassy metalsAmorphous metalAn amorphous metal is a metallic material with a disordered atomic-scale structure. In contrast to most metals, which are crystalline and therefore have a highly ordered arrangement of atoms, amorphous alloys are non-crystalline...
, with extremely high strength and corrosionCorrosionCorrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
resistance - eutectic alloys of sodiumSodiumSodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and potassiumPotassiumPotassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
(NaKNaKNaK, or sodium-potassium alloy, an alloy, of potassium , and sodium , is usually liquid at room temperature. Various commercial grades are available. NaK is highly reactive with water and may catch fire when exposed to air, so must be handled with special precautions...
) that are liquid at room temperature and used as coolantCoolantA coolant is a fluid which flows through a device to prevent its overheating, transferring the heat produced by the device to other devices that use or dissipate it. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, and chemically inert, neither causing nor...
in experimental fast neutron nuclear reactorFast neutron reactorA fast neutron reactor or simply a fast reactor is a category of nuclear reactor in which the fission chain reaction is sustained by fast neutrons...
s.
Others
Sodium chlorideSodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
form a eutectic mixture. It has a eutectic point of −21.2 C and 23.3% salt by mass. The eutectic nature of salt and water is exploited when salt is spread on roads to aid snow removal
Snow removal
Snow removal is the job of removing snow after a snowfall to make travel easier and safer. This is done by both individual households and by governments and institutions.-De-icing and anti-icing:...
, or mixed with ice to produce low temperatures (for example, in traditional ice cream
Ice cream
Ice cream is a frozen dessert usually made from dairy products, such as milk and cream, and often combined with fruits or other ingredients and flavours. Most varieties contain sugar, although some are made with other sweeteners...
making).
60% NaNO3 and 40% KNO3 forms a eutectic mixture which is used in solar molten salt technology.. To reduce the eutectic melting point in the solar molten salts the Calcium nitrate
Calcium nitrate
Calcium nitrate, also called Norgessalpeter , is the inorganic compound with the formula Ca2. This colourless salt absorbs moisture from the air and is commonly found as a tetrahydrate. It is mainly used as a component in fertilizers but is found other applications...
is used in this proportion, 42% Ca(NO3)2, 43% KNO3 and 15% NaNO3.
Lidocaine
Lidocaine
Lidocaine , Xylocaine, or lignocaine is a common local anesthetic and antiarrhythmic drug. Lidocaine is used topically to relieve itching, burning and pain from skin inflammations, injected as a dental anesthetic or as a local anesthetic for minor surgery.- History :Lidocaine, the first amino...
and prilocaine
Prilocaine
Prilocaine is a local anesthetic of the amino amide type first prepared by Claes Tegner and Nils Lofgren. In its injectable form , it is often used in dentistry. It is also often combined with lidocaine as a preparation for dermal anesthesia , for treatment of conditions like paresthesia...
, both solids at room temperature, form a eutectic that is an oil with a 16 °C (60.8 °F) melting point, used in eutectic mixture of local anesthetic (EMLA) preparations.
Mineral
Mineral
A mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties. By comparison, a rock is an aggregate of minerals and/or mineraloids and does not...
s may form eutectic mixtures in igneous rocks, giving rise to characteristic intergrowth textures such as that of granophyre
Granophyre
Granophyre is a subvolcanic rock that contains quartz and alkali feldspar in characteristic angular intergrowths such as those in the accompanying image....
.
Some inks are eutectic mixtures, allowing inkjet printer
Inkjet printer
An inkjet printer is a type of computer printer that creates a digital image by propelling droplets of ink onto paper. Inkjet printers are the most commonly used type of printer and range from small inexpensive consumer models to very large professional machines that can cost up to thousands of...
s to operate at lower temperatures.
Eutectoid

Ferrite (iron)
Ferrite or alpha iron is a materials science term for iron, or a solid solution with iron as the main constituent, with a body centred cubic crystal structure. It is the component which gives steel and cast iron their magnetic properties, and is the classic example of a ferromagnetic material...
and cementite, often in lamellar structures such as pearlite
Pearlite
Pearlite is often said to be a two-phased, lamellar structure composed of alternating layers of alpha-ferrite and cementite that occurs in some steels and cast irons...
and bainite
Bainite
Bainite is an acicular microstructure that forms in steels at temperatures from approximately 250-550°C . First described by E. S. Davenport and Edgar Bain, it is one of the decomposition products that may form when austenite is cooled past a critical temperature of 727 °C...
. This eutectoid point occurs at 723 °C (1,333.4 °F) and about 0.83% carbon.
Peritectoid
A peritectoid transformation is a type of isothermal reversible reactionReversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
that have two solid phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
s reacting with each other upon cooling of a binary, ternary, ... ,
 alloy
alloyAlloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
to create a completely different and single solid phase. The reaction plays a key role in the order and decomposition
Decomposition
Decomposition is the process by which organic material is broken down into simpler forms of matter. The process is essential for recycling the finite matter that occupies physical space in the biome. Bodies of living organisms begin to decompose shortly after death...
of quasicrystalline phases in several alloy types.
Peritectic
Peritectic transformations are also similar to eutectic reactions. Here, a liquid and solid phase of fixed proportions react at a fixed temperature to yield a single solid phase. Since the solid product forms at the interface between the two reactants, it can form a diffusion barrier and generally causes such reactions to proceed much more slowly than eutectic or eutectoid transformations. Because of this, when a peritectic composition solidifies it does not show the lamellar structure that is found with eutectic solidification.Such a transformation exists in the iron-carbon system, as seen near the upper-left corner of the figure. It resembles an inverted eutectic, with the δ phase combining with the liquid to produce pure austenite at 1495 °C (2,723 °F) and 0.17% carbon.

