Fast ion conductor
Encyclopedia
In solid-state ionics
, fast ion conductors, also known as solid electrolytes and superionic conductors, are materials that act as solid state
ion conductors and are used primarily in solid oxide fuel cells. As solid electrolytes they conduct due to the movement of ions through voids, or empty crystallographic positions, in their crystal lattice structure. The most commonly used solid electrolyte is yttria-stabilized zirconia
, YSZ. One component of the structure, the cation or anion, is essentially free to move throughout the structure, acting as charge carrier
.
The important case of fast ionic conduction is one in a surface space-charge layer of ionic crystals. Such conduction was first predicted by Kurt Lehovec
.
As a space-charge layer has nanometer thickness, the effect is directly related to nanoionics
(nanoionics-I). Lehovec’s effect is used as a basis for developing nanomaterials
for portable lithium batteries and fuel cells.
Fast ion conductors are intermediate in nature between crystal
line solids which possess a regular structure with immobile ions, and liquid electrolytes which have no regular structure and fully mobile ions. Solid electrolytes find use in all solid state supercapacitor
s, batteries and fuel cell
s, and in various kinds of chemical sensors.
s are a special class of solid electrolytes, where hydrogen ion
s act as charge carriers.
There is difference between solid electrolytes and superionic conductors.
In solid electrolytes (glasses or crystals), the ionic conductivity Ωi is an arbitrary value but it should be greatly larger than electronic one. Usually, the solids where electronic conductivity Ωe is arbitrary value but Ωi is an order of 0.0001-0.1 Ohm−1 cm−1 (300 K) are called superionic conductors.
Superionic conductors, where Ωi is more than 0.1 Ohm−1 cm−1 (300 K) and activation energy for ion transport Ei is small (about 0.1 eV), are called by advanced superionic conductors. The famous example of advanced superionic conductor-solid electrolyte is RbAg4I5
where Ωi > 0.25 Ohm−1 cm−1 and Ωe ~10−9 Ohm−1 cm−1 at 300 K. The Hall (drift) ionic mobility in RbAg4I5 is about 2x10−4 cm2/(V•s) at room temperatures.
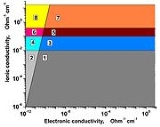
The Ωe – Ωi systematic diagram distinguishing the different types of solid state ionic conductors is given on the figure
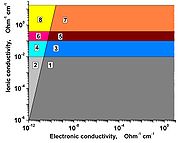 Fig. Classification of solid state ionic conductors by the lg Ωe - lg Ωi diagram (Ohm−1 cm−1).
Fig. Classification of solid state ionic conductors by the lg Ωe - lg Ωi diagram (Ohm−1 cm−1).
2, 4 and 6 – known solid electrolytes (SEs), materials
with Ωi >> Ωe;
1, 3, and 5 – known mixed ion-electron conductors;
3 and 4 – superionic conductors (SICs), i.e. materials
with Ωi > 0.001 Ohm−1cm−1, Ωe – arbitrary value;
4 – SIC and simultaneously SE, Ωi > 0.001 Ohm−1cm−1,
Ωi >>Ωe;
5 and 6 – advanced superionic conductors (AdSICs),
where Ωi > 10−1 Ohm−1cm−1 (300 K),
energy activation Ei about 0.1 eV, Ωe – arbitrary value;
6 – AdSIC and simultaneously SE, Ωi > 10−1 Ohm−1cm−1,
Ei about 0.1 eV, Ωi >>Ωe;
7 and 8 – hypothetical AdSIC with Ei ≈ kBT ≈0.03 eV (300 К);
8 – hypothetical AdSIC and simultaneously SE.
, beta-lead fluoride, zirconium dioxide
, silver iodide
.
Solid-state ionics
Solid-state ionics is the study of solid electrolytes and their uses. Some materials that fall into this category include inorganic crystalline and polycrystalline solids, ceramics, glasses, polymers, and composites...
, fast ion conductors, also known as solid electrolytes and superionic conductors, are materials that act as solid state
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
ion conductors and are used primarily in solid oxide fuel cells. As solid electrolytes they conduct due to the movement of ions through voids, or empty crystallographic positions, in their crystal lattice structure. The most commonly used solid electrolyte is yttria-stabilized zirconia
Yttria-stabilized zirconia
Yttria-stabilized zirconia is a zirconium-oxide based ceramic, in which the particular crystal structure of zirconium oxide is made stable at room temperature by an addition of yttrium oxide...
, YSZ. One component of the structure, the cation or anion, is essentially free to move throughout the structure, acting as charge carrier
Charge carrier
In physics, a charge carrier is a free particle carrying an electric charge, especially the particles that carry electric currents in electrical conductors. Examples are electrons and ions...
.
The important case of fast ionic conduction is one in a surface space-charge layer of ionic crystals. Such conduction was first predicted by Kurt Lehovec
Kurt Lehovec
Kurt Lehovec is one of the pioneers of the integrated circuit, 1959. He innovated the concept of p-n junction isolation used in every circuit element with a guard ring: a reverse-biased p-n junction surrounding the planar periphery of that element. This patent was assigned to Sprague Electric...
.
As a space-charge layer has nanometer thickness, the effect is directly related to nanoionics
Nanoionics
Nanoionics is the study and application of phenomena, properties, effects and mechanisms of processes connected with fast ion transport in all-solid-state nanoscale systems. The topics of interest include fundamental properties of oxide ceramics at nanometer length scales, and fast ion conductor...
(nanoionics-I). Lehovec’s effect is used as a basis for developing nanomaterials
Nanomaterials
Nanomaterials is a field that takes a materials science-based approach to nanotechnology. It studies materials with morphological features on the nanoscale, and especially those that have special properties stemming from their nanoscale dimensions...
for portable lithium batteries and fuel cells.
Fast ion conductors are intermediate in nature between crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
line solids which possess a regular structure with immobile ions, and liquid electrolytes which have no regular structure and fully mobile ions. Solid electrolytes find use in all solid state supercapacitor
Supercapacitor
An electric double-layer capacitor , also known as supercapacitor, supercondenser, electrochemical double layer capacitor, or ultracapacitor, is an electrochemical capacitor with relatively high energy density. Their energy density is typically hundreds of times greater than conventional...
s, batteries and fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
s, and in various kinds of chemical sensors.
Classification
Proton conductorProton conductor
A proton conductor is an electrolyte, typically a solid electrolyte, in which H+ are the primary charge carriers.-Composition:For practical applications, proton conductors are usually solid materials. Typical materials are polymers or ceramic. Typically the pores in practical materials are small...
s are a special class of solid electrolytes, where hydrogen ion
Hydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
s act as charge carriers.
There is difference between solid electrolytes and superionic conductors.
In solid electrolytes (glasses or crystals), the ionic conductivity Ωi is an arbitrary value but it should be greatly larger than electronic one. Usually, the solids where electronic conductivity Ωe is arbitrary value but Ωi is an order of 0.0001-0.1 Ohm−1 cm−1 (300 K) are called superionic conductors.
Superionic conductors, where Ωi is more than 0.1 Ohm−1 cm−1 (300 K) and activation energy for ion transport Ei is small (about 0.1 eV), are called by advanced superionic conductors. The famous example of advanced superionic conductor-solid electrolyte is RbAg4I5
Rubidium silver iodide
Rubidium silver iodide is a ternary inorganic compound with the formula RbAg4I5. It is an unusual solid where the electrical conductivity involves movement of silver ions within the crystal lattice...
where Ωi > 0.25 Ohm−1 cm−1 and Ωe ~10−9 Ohm−1 cm−1 at 300 K. The Hall (drift) ionic mobility in RbAg4I5 is about 2x10−4 cm2/(V•s) at room temperatures.
The Ωe – Ωi systematic diagram distinguishing the different types of solid state ionic conductors is given on the figure

2, 4 and 6 – known solid electrolytes (SEs), materials
with Ωi >> Ωe;
1, 3, and 5 – known mixed ion-electron conductors;
3 and 4 – superionic conductors (SICs), i.e. materials
with Ωi > 0.001 Ohm−1cm−1, Ωe – arbitrary value;
4 – SIC and simultaneously SE, Ωi > 0.001 Ohm−1cm−1,
Ωi >>Ωe;
5 and 6 – advanced superionic conductors (AdSICs),
where Ωi > 10−1 Ohm−1cm−1 (300 K),
energy activation Ei about 0.1 eV, Ωe – arbitrary value;
6 – AdSIC and simultaneously SE, Ωi > 10−1 Ohm−1cm−1,
Ei about 0.1 eV, Ωi >>Ωe;
7 and 8 – hypothetical AdSIC with Ei ≈ kBT ≈0.03 eV (300 К);
8 – hypothetical AdSIC and simultaneously SE.
Examples
Examples of fast ion conductors include beta-alumina solid electrolyteBeta-alumina solid electrolyte
Beta-alumina solid electrolyte is a fast ion conductor material used as a membrane in several types of molten salt electrochemical cell...
, beta-lead fluoride, zirconium dioxide
Zirconium dioxide
Zirconium dioxide , sometimes known as zirconia , is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the rare mineral baddeleyite. The high temperature cubic crystalline form is rarely found in nature as mineral tazheranite O2...
, silver iodide
Silver iodide
Silver iodide is a yellow, inorganic, photosensitive iodide of silver used in photography, in medicine as an antiseptic, and in rainmaking for cloud seeding.-Crystal structure:...
.
- Inorganic materials:
- Zirconium dioxideZirconium dioxideZirconium dioxide , sometimes known as zirconia , is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the rare mineral baddeleyite. The high temperature cubic crystalline form is rarely found in nature as mineral tazheranite O2...
doped with calcium oxideCalcium oxideCalcium oxide , commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline crystalline solid at room temperature....
and yttrium oxide, which is conductive for O2-OxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
ions and is used in oxygen sensorOxygen sensorAn oxygen sensor, or lambda sensor, is an electronic device that measures the proportion of oxygen in the gas or liquid being analyzed. It was developed by the Robert Bosch GmbH company during the late 1960s under the supervision of Dr. Günter Bauman...
s - Beta-alumina solid electrolyteBeta-alumina solid electrolyteBeta-alumina solid electrolyte is a fast ion conductor material used as a membrane in several types of molten salt electrochemical cell...
used as a membrane in several types of molten salt electrochemical cells - Lanthanum(III) fluoride, conductive for F- ions, used in some ion selective electrodeIon selective electrodeAn ion-selective electrode , also known as a specific ion electrode , is a transducer that converts the activity of a specific ion dissolved in a solution into an electrical potential, which can be measured by a voltmeter or pH meter. The voltage is theoretically dependent on the logarithm of the...
s - Silver sulfideSilver sulfideSilver sulfide, Ag2S, is the sulfide of silver. This dense black solid constitutes the tarnish that forms over time on silverware and other silver objects. Silver sulfide is insoluble in all solvents, but is degraded by strong acids. Silver sulfide features a covalent bond, as it is made up of...
, conductive for Ag+ ions, used in some ion selective electrodeIon selective electrodeAn ion-selective electrode , also known as a specific ion electrode , is a transducer that converts the activity of a specific ion dissolved in a solution into an electrical potential, which can be measured by a voltmeter or pH meter. The voltage is theoretically dependent on the logarithm of the...
s - Silver iodideSilver iodideSilver iodide is a yellow, inorganic, photosensitive iodide of silver used in photography, in medicine as an antiseptic, and in rainmaking for cloud seeding.-Crystal structure:...
, conductive at higher temperatures - Copper iodide
- Beta-lead fluoride, exhibits a continuous growth of conductivity on heating. This property was first discovered by M. Faraday
- Lead(II) chlorideLead(II) chlorideLead chloride is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead chloride is one of the most important lead-based reagents...
, conductive at higher temperatures - Rubidium silver iodideRubidium silver iodideRubidium silver iodide is a ternary inorganic compound with the formula RbAg4I5. It is an unusual solid where the electrical conductivity involves movement of silver ions within the crystal lattice...
, conductive at room temperature - Some perovskitePerovskiteA perovskite structure is any material with the same type of crystal structure as calcium titanium oxide , known as the perovskite structure, or XIIA2+VIB4+X2−3 with the oxygen in the face centers. Perovskites take their name from this compound, which was first discovered in the Ural mountains of...
ceramics - strontium titanateStrontium titanateStrontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure...
, strontium stannate - conductive for O2- ions - Zr(HPO4)2.nH2O - conductive for H+ ions
- UO2HPO4.4H2O - conductive for H+ ions
- Conductive ceramicCeramicA ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
s - e.g. NASICON (Na3Zr2Si2PO12), a sodium super-ionic conductor
- Zirconium dioxide
- Organic materials:
- GelGelA gel is a solid, jelly-like material that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady-state...
s - polyacrylamidePolyacrylamidePolyacrylamide is a polymer formed from acrylamide subunits. It can be synthesized as a simple linear-chain structure or cross-linked, typically using N,N-methylenebisacrylamide. Polyacrylamide is not toxic...
s, agarAgarAgar or agar-agar is a gelatinous substance derived from a polysaccharide that accumulates in the cell walls of agarophyte red algae. Throughout history into modern times, agar has been chiefly used as an ingredient in desserts throughout Asia and also as a solid substrate to contain culture medium...
- polymer holds a solution of ion electrolyte - A salt dissolved in a polymer - e.g. lithium perchlorateLithium perchlorateLithium perchlorate is the inorganic compound with the formula LiClO4. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate.-Uses:...
in polyethylene oxide - PolyelectrolytePolyelectrolytePolyelectrolytes are polymers whose repeating units bear an electrolyte group. These groups will dissociate in aqueous solutions , making the polymers charged. Polyelectrolyte properties are thus similar to both electrolytes and polymers , and are sometimes called polysalts. Like salts, their...
s - IonomerIonomerAn ionomer is a polymer that comprises repeat units of both electrically neutral repeating units and a fraction of ionized units...
s - e.g. NafionNafionNafion is a sulfonated tetrafluoroethylene based fluoropolymer-copolymer discovered in the late 1960s by Walther Grot of DuPont. It is the first of a class of synthetic polymers with ionic properties which are called ionomers...
, a H+ conductorProton conductorA proton conductor is an electrolyte, typically a solid electrolyte, in which H+ are the primary charge carriers.-Composition:For practical applications, proton conductors are usually solid materials. Typical materials are polymers or ceramic. Typically the pores in practical materials are small...
- Gel

