Fluorescence recovery after photobleaching
Encyclopedia
Photobleaching
Photobleaching is the photochemical destruction of a fluorophore. In microscopy, photobleaching may complicate the observation of fluorescent molecules, since they will eventually be destroyed by the light exposure necessary to stimulate them into fluorescing...
(FRAP) denotes an optical technique capable of quantifying the two dimensional lateral diffusion of a molecularly thin film containing fluorescently labeled probes, or to examine single cells. This technique is very useful in biological studies of cell membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
diffusion and protein binding. In addition, surface deposition of a fluorescing phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
bilayer (or monolayer) allows the characterization of hydrophilic
Hydrophile
A hydrophile, from the Greek "water" and φιλια "love," is a molecule or other molecular entity that is attracted to, and tends to be dissolved by water. A hydrophilic molecule or portion of a molecule is one that has a tendency to interact with or be dissolved by, water and other polar substances...
(or hydrophobic
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
) surfaces in terms of surface structure and free energy.
Similar, though less well known, techniques have been developed to investigate the 3-dimensional diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
and binding
Binding (molecular)
Molecular binding is an attractive interaction between two molecules which results in a stable association in which the molecules are in close proximity to each other...
of molecules inside the cell; they are also referred to as FRAP.
Experimental Setup
The basic apparatus comprises an optical microscopeOptical microscope
The optical microscope, often referred to as the "light microscope", is a type of microscope which uses visible light and a system of lenses to magnify images of small samples. Optical microscopes are the oldest design of microscope and were possibly designed in their present compound form in the...
, a light source and some fluorescent probe. Fluorescent emission
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
is contingent upon absorption of a specific optical wavelength or color which restricts the choice of lamps. Most commonly, a broad spectrum
Spectrum
A spectrum is a condition that is not limited to a specific set of values but can vary infinitely within a continuum. The word saw its first scientific use within the field of optics to describe the rainbow of colors in visible light when separated using a prism; it has since been applied by...
mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
or xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
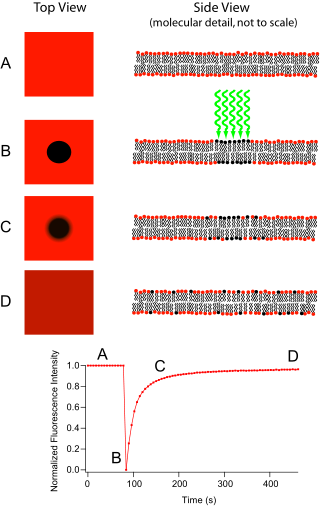
source is used in conjunction with a color filter. The technique begins by saving a background image of the sample before photobleaching. Next, the light source is focused onto a small patch of the viewable area either by switching to a higher magnification microscope objective or with laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
light of the appropriate wavelength. The fluorophores in this region receive high intensity illumination which causes their fluorescence lifetime to quickly elapse (limited to roughly 105 photons before extinction). Now the image in the microscope is that of a uniformly fluorescent field with a noticeable dark spot. As Brownian motion proceeds, the still-fluorescing probes will diffuse throughout the sample and replace the non-fluorescent probes in the bleached region. This diffusion proceeds in an ordered fashion, analytically determinable from the diffusion equation. Assuming a Gaussian
Gaussian beam
In optics, a Gaussian beam is a beam of electromagnetic radiation whose transverse electric field and intensity distributions are well approximated by Gaussian functions. Many lasers emit beams that approximate a Gaussian profile, in which case the laser is said to be operating on the fundamental...
profile for the bleaching beam, the diffusion constant D can be simply calculated from:

where w is the radius of the beam and t1/2 is the time required for the bleach spot to recover half of its initial integrated intensity.
Supported Lipid Bilayers
Originally, the FRAP technique was intended for use as a mean to characterize the mobility of individual lipid molecules within a cell membrane. While providing great utility in this role, current research leans more toward investigation of artificial lipid membranes. Supported by hydrophilic or hydrophobic substrates (to produce lipid bilayers or monolayers respectively) and incorporating membrane proteins, these biomimetic structures are potentially useful as analytical devices for determining the identity of unknown substances, understanding cellular transduction, and identifying ligand binding sites.Protein Binding
This technique is commonly used in conjunction with green fluorescent proteinGreen fluorescent protein
The green fluorescent protein is a protein composed of 238 amino acid residues that exhibits bright green fluorescence when exposed to blue light. Although many other marine organisms have similar green fluorescent proteins, GFP traditionally refers to the protein first isolated from the...
(GFP) fusion protein
Fusion protein
Fusion proteins or chimeric proteins are proteins created through the joining of two or more genes which originally coded for separate proteins. Translation of this fusion gene results in a single polypeptide with functional properties derived from each of the original proteins...
s, where the studied protein is fused to a GFP. When excited by a specific wavelength of light, the protein will fluoresce. When the protein that is being studied is produced with the GFP, then the fluorescence can be tracked. Photodestroying the GFP, and then watching the repopulation into the bleached area can reveal information about protein interaction partners, organelle continuity and protein trafficking.
If after some time the fluorescence doesn't reach the initial level anymore, then some part of the fluorescence is caused by an immobile fraction (that cannot be replenished by diffusion). Similarly, if the fluorescent proteins bind to static cell receptors, the rate of recovery will be retarded by a factor related to the association and disassociation coefficients of binding. This observation has most recently been exploited to investigate protein binding.
Applications Outside the Membrane
FRAP can also be used to monitor proteins outside the membrane. After the protein of interest is made fluorescent, generally by expression as a GFP fusion protein, a confocal microscope is used to photobleach and monitor a region of the cytoplasmCytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
, mitotic spindle
Mitotic spindle
In cell biology, the spindle fibers are the structure that separates the chromosomes into the daughter cells during cell division. It is part of the cytoskeleton in eukaryotic cells...
, nucleus
Cell nucleus
In cell biology, the nucleus is a membrane-enclosed organelle found in eukaryotic cells. It contains most of the cell's genetic material, organized as multiple long linear DNA molecules in complex with a large variety of proteins, such as histones, to form chromosomes. The genes within these...
, or another cellular structure. The mean fluorescence in the region can then be plotted versus time since the photobleaching, and the resulting curve can yield kinetic coefficients for the protein's binding reactions and/or the protein's diffusion coefficient in the medium where it is being monitored. The analysis is most simple when the curve is dominated by only the diffusional or only the binding components. For a circular bleach spot and diffusion-dominated recovery, the fluorescence is described by the Soumpasis equation and involves modified Bessel functions:

where h=r2/(2*Df*t); r=radius of bleach spot; t=time; Df=diffusion coefficient; f(t) is the normalized fluorescence (goes to 1 as t goes to infinity).
For a binding-dominated reaction, in which the diffusion is much faster than the unbinding of the bleached protein and subsequent binding of unbleached protein, it is given by

where Beq is the fraction of the protein that is bound to other structures in the photobleached region at equilibrium, and koff is the dissociation constant for the binding. Sometimes there are multiple binding states in which case there are just more exponential terms of the same form. Many FRAP recoveries are not dominated overwhelmingly by just diffusion or just binding, so their curves are more complex; FRAP recoveries are analyzed in much more detail in Sprague and Pego et al. (see References below).
See also
- Fluorescence microscopeFluorescence microscopeA fluorescence microscope is an optical microscope used to study properties of organic or inorganic substances using the phenomena of fluorescence and phosphorescence instead of, or in addition to, reflection and absorption...
- PhotobleachingPhotobleachingPhotobleaching is the photochemical destruction of a fluorophore. In microscopy, photobleaching may complicate the observation of fluorescent molecules, since they will eventually be destroyed by the light exposure necessary to stimulate them into fluorescing...
- Fluorescence loss in photobleachingFluorescence loss in photobleachingFluorescence Loss in Photobleaching, or FLIP, is a technique in fluorescence microscopy which can be used to examine the movement or diffusion of molecules inside cells or membranes. Typically a cell membrane is labelled with a fluorescent dye, and a specific area of the labeled membrane is...
(FLIP)

