
Fulvalene
Encyclopedia
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
obtained by formally cross-conjugating
Cross-conjugation
Cross-conjugation is a special type of conjugation in a molecule, when in a set of three Pi bonds only two pi-bonds interact with each other by conjugation, the third one is excluded from interaction...
two rings
Cycloalkane
Cycloalkanes are types of alkanes that have one or more rings of carbon atoms in the chemical structure of their molecules. Alkanes are types of organic hydrocarbon compounds that have only single chemical bonds in their chemical structure...
through a common exocyclic double bond
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
. The name is derived from the similarly structured fulvene
Fulvene
Fulvene is one of several hydrocarbons with the same formula as benzene, C6H6. Fulvenes include the derivatives of this simple hydrocarbon, which itself is rarely encountered. Thiele is credited with discovering the scope of the reaction between cyclopentadiene and aldehydes and ketones that...
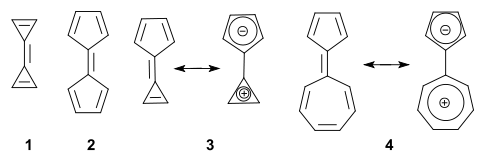
s which lack one ring. Triapentafulvalene (3) is also known as calicene as in calix or chalice
Chalice (cup)
A chalice is a goblet or footed cup intended to hold a drink. In general religious terms, it is intended for drinking during a ceremony.-Christian:...
because of its wine-glass appearance.
In general, the parent fulvalenes are very unstable and for instance the parent triafulvalene (1) has not even been synthesized. On the other hand stable fulvalenes can be obtained by proper substitution
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
or benzannulation. Several members should be stabilized taking into account a dipolar mesomeric form with for instance pentaheptafulvalene 4, which can be thought of as a tropylium ion
Tropylium ion
In organic chemistry, the tropylium ion is an aromatic species with a formula of [C7H7]+. Its name derives from the molecule tropine . Salts of the tropylium cation can be stable, e.g. tropylium tetrafluoroborate...
joined to a cyclopentadienyl
Cyclopentadienyl
In organic chemistry, cyclopentadienyl is a cyclic group of atoms with the formula C5H5. Cyclopentadienyl are closely related to cyclopentadiene. Cyclopentadienyl have five carbon atoms bonded together in a pentagonal planar ring, all five of which are bonded to individual hydrogen atoms...
anion (both stable and aromatic). In this compound the dipolar structure is calculated to contribute 23% to the total structure.
Pentafulvalene
Pentafulvalene is the member of the fulvalene family with the molecular formula C10H8. It is of theoretical interest as one of the simplest non-benzenoid conjugatedConjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s. Fulvalene is an unstable isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
of the more common benzenoid aromatic compounds naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
and azulene
Azulene
Azulene is an organic compound and an isomer of naphthalene. Whereas naphthalene is colourless, azulene is dark blue. Its name is derived from the Spanish word azul, meaning "blue"...
. It is also known as bicyclopentadienylidene. Pentafulvalene consists of two 5-membered rings, each with two double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s, joined by yet a fifth double bond. It has D2h symmetry
Molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
.
An earlier attempt at synthesis of pentafulvalene in 1951 by Pauson and Kealy resulted in the accidental discovery of ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
. Its synthesis was first reported in 1958 by E. A. Matzner at Yale University
Yale University
Yale University is a private, Ivy League university located in New Haven, Connecticut, United States. Founded in 1701 in the Colony of Connecticut, the university is the third-oldest institution of higher education in the United States...
, working under William von Eggers Doering
William von Eggers Doering
William von Eggers Doering was a Professor Emeritus at Harvard University and the former Chair of its Chemistry Department...
. In this method, dissertation only and never published, a cyclopentadienyl anion is coupled with iodine to the dihydrofulvalene which is then doubly deprotonated
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
(n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
) to the dianion and then oxidized with oxygen. Pentafulvalene was spectroscopically observed at 77 K
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
from photolysis of diazocyclopentadiene (dimerization of two cyclopentadiene carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s) with UV spectra matching those obtained by the Doering group. Final isolation of the compound came in 1984 via a method similar to that of Doering. The compound was found to be nonaromatic and extremely reactive above −50 °C through Diels-Alder dimerization
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
.
Perchlorofulvalene C10Cl8 is quite stable in contrast to the hydrocarbon. Tetrathiafulvalene
Tetrathiafulvalene
Tetrathiafulvalene is a organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, 2, by replacement of four CH groups with sulfur atoms...
is an organic semiconductor
Organic semiconductor
An organic semiconductor is an organic material with semiconductor properties. Single molecules, short chain and organic polymers can be semiconductive. Semiconducting small molecules include the polycyclic aromatic compounds pentacene, anthracene, and rubrene...
.
Fulvalenes as a ligand
Fulvalenes forms stable organometallic complexes that can be formally considered derivatives of the dianion C10H82

