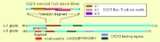
Gluten immunochemistry
Encyclopedia
The immunochemistry
of Triticeae glutens
is important in several inflammatory diseases. It can be subdivided into innate responses (direct stimulation of immune system), class II
mediated presentation (HLA DQ), class I
meditiated stimulation of killer cells, and antibody
recognition. The responses to gluten
protein
s and polypeptide regions differs according to the type of gluten sensitivity. The response is also dependent on the genetic makeup of the human leukocyte antigen
genes. In gluten sensitive enteropathy, there are 4 types of recognition, innate immunity (a form of cellular immunity priming), HLA-DQ
, and antibody
recognition of gliadin and transglutaminase. With idiopathic gluten sensitivity only antibody recognition to gliadin has been resolved. In wheat allergy
, the response pathways are mediated through IgE against other wheat proteins and other forms of gliadin.

Innate immunity to gluten
refers to an immune response that works independently of T-cell receptor or antibody
recognition of the 'innate' peptide. This peptide acts directly on cells, such as monocytes, stimulating their growth and differentiation. Innate immunity to gluten is complicated by an apparent role gluten has in bypassing normal host defense and peptide exclusion mechanisms in the gut. While not truly innate, these activities allow gliadin to enter into areas where many lymphocytes patron. In bypassing these filters gliadin alter the normal behavior of both digestive cells, called enterocytes or epithelial cells, and lymphocytes. This increases the potential of causing sensitivity (see Underlying Conditions). One potential explanation of why certain people become sensitive is that these individuals may not produce adequate peptidases in some areas of the gut, allowing these peptides to survive. Other explanation for some may be that food chemicals or drugs are weakening the defenses. This can be the case with ω5-gliadin allergy with salicylate sensitivity. There is no clear reasoning, either from genetics or from long term studies of susceptible individuals why these gut peptide restrictions would change.
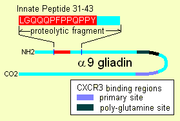 Once inside, α-9 gliadin 31-55 shows the ability to activate undifferentiated immune cells that then proliferate and also produce inflammatory hormones notably Interleukin 15. This produces a number of downstream responses that are pro-inflammatory. The other peptide that may have innate behavior is the "CXCR3" receptor binding peptides, the receptor exists on enterocytes, the brush border membrane cells. The peptide displaces an immune factor and signals the disruption of the membrane seal, the tight junctions, between cells.
Once inside, α-9 gliadin 31-55 shows the ability to activate undifferentiated immune cells that then proliferate and also produce inflammatory hormones notably Interleukin 15. This produces a number of downstream responses that are pro-inflammatory. The other peptide that may have innate behavior is the "CXCR3" receptor binding peptides, the receptor exists on enterocytes, the brush border membrane cells. The peptide displaces an immune factor and signals the disruption of the membrane seal, the tight junctions, between cells.
s. The 25mer is also resistant to Brush border
membrane peptidases of the small intestine
in coeliacs. IRP induced the rapid expression of interleukin 15
(IL15) and other factors. Thus IRP activates the immune system. Studies show that, while in normal individuals the peptide is trimmed over time to produce inactive peptide, in celiacs a 19mer may lose a residue from one end or the other, after prolonged incubation that 50% remains intact.
s versus normal helper T-cells. One hypothesis is that IL-15 induces the highly inflammatory Th1 response that activates T-helper cells (DQ2 restricted gliadin specific) that then orchestrate the destructive response, but the reason why inflammatory cells develop prior to gliadin specific helper cells is not known. The IRP response differs from typical responses that stimulate IL15 release, such as viral infection
. In addition, other cytokines such as IL12
and IL2
, which are typically associated with T-helper cell stimulation, are not involved. In these two ways the innate peptide activation of T-cells in celiac disease is strange. IL-15 appears to induce increases in MICA and NKG2D that may increase brush-border cell killing.
In addition, innate immunity to IRP peptide is involved in coeliac disease
, dermatitis herpetiformis
and possibly juvenile diabetes. IRP targets monocyte
s and increases the production of IL-15 by an HLA-DQ independent pathway, a subsequent study showed that both this region and the "33mer" could create the same response, in cells from both treated coeliacs and non-coeliacs. However, unlike the non-coeliacs, the treated coeliac cells produce the disease marker nitrite
. This indicates that another abnormality in people with coeliac disease that allows stimulation to proceed past the normal healthy state. After extensive study, there is no known genetic association for this that appears to stand out at present, and implicates other environmental factors in the defect.
and Zonulin
expression. The factor it displaces, I-TAC, is a T-cell attractant. This peptide may also be involved in increased risk for type 1 diabetes as zonulin
production is also a factor. This triggering of zonulin ultimately results in the degradation of tight junctions allowing large solutes, such as proteolytic resistant gliadin fragments to enter behind the brush border membrane cells.
One study examined the effect of ω-5 gliadin, the primary cause of WD-EIA, and found increased permeability of intestinal cells. Other studies show that IgE reactivity to ω-5 gliadin increases greatly when deamidated or crosslinked to transglutaminase.
presentation has been investigated.
The HLA-A antigens can mediate apoptosis
in autoimmune disease and HLA A*0201 in with the HLA-DQ8
haplotypes has been documented. The class I sites were found on the carboxyl end of gliadin at positions 123-131, 144-152, and 172-180. The involvement of class I responses may be minor, since antibodies to transglutaminase correlate with pathogenesis and recognition of extracellular matrix and cell surface transglutaminase can explain the destruction within coeliac disease. This process involves antibody-dependent cellular cytotoxicity
. With regard to a receptor called FOS, euphemistically called the "Death Receptor", enterocytes appear to overexpress the recept in coeliac lesions, there is speculation that Class I presentation of glaidin, tTG or other peptides that invokes signalling. The role of class I receptor in cell-mediated programmed cell (enterocyte) death is not known.
HLA-DQ
proteins present polypeptide regions of proteins of about 9 amino acids and larger in size (10 to 14 residues in involved in binding is common for gliadin) to T lymphocytes.
Gliadin proteins can be adsorbed by APC. After digestion in the lysozomes of APCs, glaidin peptides can be recycled to the cell's surface bound to DQ, or they can be bound and presented directly from the cell surface. The major source of inflammatory gluten is dietary gluten. Optimal reactivity of gliadin occurs when the protein is partially digested by small intestinal lysozyme
and trypsin
into proteolytic digests. These polypeptides of gluten can then make their way behind the epithelial layer of cells (membrane), where APCs and T-cells reside in the lamina propria
. (See: Underlying conditions)
The APC bearing DQ-gliadin peptide on the surface can bind to T-cells that have an antibody-like T-cell receptor the specifically recognized DQ2.5 with gliadin. The complex (APC-DQ-glaidin) thus stimulates the gliadin specific T-cells to divide. These cells cause B-cells that recognize gliadin to proliferate. The B-cells mature into plasma cell
s producing anti-gliadin antibodies
. This does not cause coeliac disease and is an unknown factor in idiopathic disease. Enteropathy is believed to occur when tissue transglutaminase
(tTG) covelantly links itself to gliadin
peptide
s that enter the lamina propria
of the intestinal villus. The resulting structure can be presented by APC (with the same gliadin recognizing DQ isoforms) to T-cells, and B-cells can produce anti-transglutaminase antibodies
. This appears to result in the destruction of the villi. The release of gliadin by transglutaminase does not lessen disease. When tTG-gliadin undergoes hydrolysis (steals a water to cut the two apart), the result is deamidated gliadin. Deamidated gliadin peptides are more inflammatory relative to natural peptides. Deamidated gliadin is also found in foods that have added gluten, such as wheat bread, food pastes.
The major gluten proteins that are involved in coeliac disease are the α-gliadin isoforms. Alpha gliadin is composed of repeated motifs that, when digested, can be presented by HLA-DQ molecules. DQ2.5 recognizes several motifs in gluten proteins, and therefore HLA-DQ can recognize many motifs on each gliadin (see Understanding DQ haplotypes and DQ isoforms on the right) However, numbers of different proteins from the grass tribe Triticeae
have been found to carry motifs presented by HLA DQ2.5 and DQ8. Wheat
has a large number of these proteins because its genome contains chromosomes derived from two goat grass
species and a primitive wheat species. The positions of these motifs in different species, strains and isoforms may vary because of insertions and deletions in sequence. There are a large number of wheat variants, and a large number of gliadin
s in each variant, and thus many potential sites. These proteins once identified and sequenced can be surveyed by sequence homology searches.
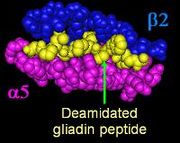
HLA-DQ2
primarily presents gliadins with the HLA-DQ isoform DQ2.5 (DQ α5-β2) isoform. DQA1*0202:DQB1*0201 homozygotes (DQ α2-β2) also appear to be able to present pathogenic gliadin peptides, but a smaller set with lower binding affinity.
and therefore can be modified by deamidation in the gut to create more inflammatory peptides. The most important recognition appears to be directed toward the α-/β-gliadin
s. An example of the repetition of a motif across many proteins, the α-2 gliadin (57-68) and (62-75) are also found on α-4, α-9 gliadin. Many gliadins contain the "α-20 motif", which is found in wheat and other Triticeae genera.(see also: "α-20" gliadin motifs). Alpha-2 secalin
, the glutinous protein in rye, is composed of two amino-terminal overlapping T-cell sites at positions (8-19) and (13-23).
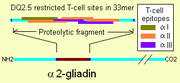
. Α2-gliadin differs from the other α-gliadins, specifically because it contains an insert of 14 amino acids. This particular insertion creates 6 T-cell sites where, in the most similar gliadins, there are 2 or less sites. The sites belong to three epitope groups "α-I", "α-II", and "α-III" The insertion also creates a larger region of α-gliadin that is resistant to gastrointestinal proteases. The smallest digest of trypsin and chymotrypsin for the region is a 33mer. This particular region has three tissue transglutaminase sites, two sites that lie within the 14 amino acid insertion, a region of maximal stimulation are found with more than 80% reduction in response for native, un-deaminated, sequence at the position. Because of the density of T-cell sites on the "33mer" the affinity for deamidated gliadin is strongly indicates that it may be best treated as a single T-cell site of much higher affinity. This site alone may fulfill all the T-helper cell adaptive immune requirements with HLA-DQ2.5 involvement in some coeliac disease.
The gamma epitopes identified are DQ2-"γ-I", -"γ-II" (γ30), -"γ-III", -"γ-IV", -"γ-VI" and -"γ-VII"
Some of these epitopes are recognized in children who do not have T-cell reactivities toward α-2 gliadin. A 26 residue proteolytic resistance fragment has been found on γ-5 gliadin, positions 26–51, that has multiple transglutaminase and T-cell epitopes. This site has 5 overlapping T-cells sites of DQ2-"γ-II", -"γ-III", -"γ-IV", and "γ-glia 2". Computer analysis of 156 prolamins and glutelins revealed many more resistant fragments, one , a γ-gliadin, containing 4 epitopes was 68 amino acids in length.
s of other Triticeae
genera, a gliadin that appears to similar to ancestral. Antigen presenting cells bearing DQ2.2 can present alpha gliadin sites, for example alpha-II region of the "33mer" and therefore the "33mer" may have a role in DQ2.2 bearing individuals, but the binding capacity is substantially lower.
confers susceptibility to coeliac disease but in a fashion somewhat similar to DQ2.5. Homozygotes of DQ8, DQ2.5/DQ8 and DQ8/DQ2.2 are higher than expected based on levels in the general population.(see: Understanding DQ haplotypes and DQ isoforms). HLA-DQ8 is generally not as involved in the most severe complications, and it does not recognize the "33mer" of α-2 gliadin to the same degree as DQ2.5. There are a smaller number of gliadin (prolamin) peptides presented by HLA-DQ8. A few studies have been done on the adaptive immune response for DQ8/DQ2- individuals. DQ8 appears to rely much more on adaptive immunity to the carboxyl half of alpha gliadins. In addition, it appear to react with gamma gliadin to a degree comparable to DQ2.5. T-cell responses to the high molecular weight glutenin may be more important with DQ8 mediated than DQ2.5 mediated celiac disease.
has an ambiguous pathogenesis in coeliac disease. The crosslinking of gliadin with tissue transglutaminase leads to the production of anti-transglutaminase antibodies
, but this is mediated through T-cell recognition of gliadin. The allergic recognition of gliadin
by mast cells, eosinophiles in the presence of IgE has notable direct consequences, such as exercise-induced anaphylaxis
.
Anti-gliadin antibodies, like those detected in celiac disease bind to the α-2 gliadin(57-73).
This site is within the T-cell reactive "33mer" presented by DQ2.5. There has been some suggestion wheat plays a role in juvenile diabetes as antibodies to the non-glutinous seed storage glb-1 (a globulin) are implicated in crossreactive autoantigenic antibodies that destroy islet cells in the pancreas. Anti-gliadin antibodies have been found to synapsin I
Omega-gliadin and the HMW Glutenin subunit antibodies have been found most commonly in individuals with exercise-induced anaphylaxis
and Baker's allergy, and represent a potent class of gluten allergens. Non-glutinous proteins in wheat are also allergens, these include: LTP (albumin
/globulin
), thioredoxin
-hB, and wheat flour peroxidase
. A particular 5 residue peptide, Gln-Gln-Gln-Pro-Pro motif, has been found to be a major wheat allergen.
Immunochemistry
Immunochemistry is a branch of chemistry that involves the study of the reactions and components on the immune system.Various methods in immunochemistry have been developed and refined, and been used in scientific study, from virology to molecular evolution....
of Triticeae glutens
Triticeae glutens
Triticeae glutens are seed storage proteins found in mature seeds of grass tribe Triticeae. Seed glutens of non-Triticeae plants have varieties of similar properties, but none singly can perform on a par with those of the Triticeae taxa, particularly the triticum species...
is important in several inflammatory diseases. It can be subdivided into innate responses (direct stimulation of immune system), class II
MHC class II
MHC Class II molecules are found only on a few specialized cell types, including macrophages, dendritic cells and B cells, all of which are professional antigen-presenting cells ....
mediated presentation (HLA DQ), class I
MHC class I
MHC class I molecules are one of two primary classes of major histocompatibility complex molecules and are found on every nucleated cell of the body...
meditiated stimulation of killer cells, and antibody
Antibody
An antibody, also known as an immunoglobulin, is a large Y-shaped protein used by the immune system to identify and neutralize foreign objects such as bacteria and viruses. The antibody recognizes a unique part of the foreign target, termed an antigen...
recognition. The responses to gluten
Gluten
Gluten is a protein composite found in foods processed from wheat and related grain species, including barley and rye...
protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s and polypeptide regions differs according to the type of gluten sensitivity. The response is also dependent on the genetic makeup of the human leukocyte antigen
Human leukocyte antigen
The human leukocyte antigen system is the name of the major histocompatibility complex in humans. The super locus contains a large number of genes related to immune system function in humans. This group of genes resides on chromosome 6, and encodes cell-surface antigen-presenting proteins and...
genes. In gluten sensitive enteropathy, there are 4 types of recognition, innate immunity (a form of cellular immunity priming), HLA-DQ
HLA-DQ
HLA-DQ is a cell surface receptor type protein found on antigen presenting cells. DQ is an αβ heterodimer of the MHC Class II type. The α and β chains are encoded by HLA-DQA1 and HLA-DQB1, respectively. These two loci are adjacent to each other on chromosome 6p21.3. Both the α-chain and β-chain...
, and antibody
Antibody
An antibody, also known as an immunoglobulin, is a large Y-shaped protein used by the immune system to identify and neutralize foreign objects such as bacteria and viruses. The antibody recognizes a unique part of the foreign target, termed an antigen...
recognition of gliadin and transglutaminase. With idiopathic gluten sensitivity only antibody recognition to gliadin has been resolved. In wheat allergy
Wheat allergy
Wheat allergy is a food allergy, but can also be a contact allergy resulting from occupational exposure. Like all allergies wheat allergy involves IgE and mast cell response. Typically the allergy is limited to the seed storage proteins of wheat, some reactions are restricted to wheat proteins,...
, the response pathways are mediated through IgE against other wheat proteins and other forms of gliadin.

Innate immunity
EWLINE
|
Innate immunity to gluten
Gluten
Gluten is a protein composite found in foods processed from wheat and related grain species, including barley and rye...
refers to an immune response that works independently of T-cell receptor or antibody
Antibody
An antibody, also known as an immunoglobulin, is a large Y-shaped protein used by the immune system to identify and neutralize foreign objects such as bacteria and viruses. The antibody recognizes a unique part of the foreign target, termed an antigen...
recognition of the 'innate' peptide. This peptide acts directly on cells, such as monocytes, stimulating their growth and differentiation. Innate immunity to gluten is complicated by an apparent role gluten has in bypassing normal host defense and peptide exclusion mechanisms in the gut. While not truly innate, these activities allow gliadin to enter into areas where many lymphocytes patron. In bypassing these filters gliadin alter the normal behavior of both digestive cells, called enterocytes or epithelial cells, and lymphocytes. This increases the potential of causing sensitivity (see Underlying Conditions). One potential explanation of why certain people become sensitive is that these individuals may not produce adequate peptidases in some areas of the gut, allowing these peptides to survive. Other explanation for some may be that food chemicals or drugs are weakening the defenses. This can be the case with ω5-gliadin allergy with salicylate sensitivity. There is no clear reasoning, either from genetics or from long term studies of susceptible individuals why these gut peptide restrictions would change.

Alpha gliadin 31-43
Gluten bears an innate response peptide (IRP) found on α-9 gliadin, at positions 31-43 and on α-3, 4, 5, 8, and 11 gliadins. The IRP lies within a 25 amino-acid long region that is resistant to pancreatic proteaseExocrine pancreas
The exocrine pancreas has ducts that are arranged in clusters called acini . Pancreatic secretions are secreted into the lumen of the acinus, and then accumulate in intralobular ducts that drain to the main pancreatic duct, which drains directly into the duodenum.Control of the exocrine function of...
s. The 25mer is also resistant to Brush border
Brush border
A brush border is the name for the microvilli-covered surface of simple cuboidal epithelium and simple columnar epithelium cells found in certain locations of the body. Microvilli are approximately 100 nanometers in diameter and their length varies from approximately 100 to 2,000 nanometers in...
membrane peptidases of the small intestine
Small intestine
The small intestine is the part of the gastrointestinal tract following the stomach and followed by the large intestine, and is where much of the digestion and absorption of food takes place. In invertebrates such as worms, the terms "gastrointestinal tract" and "large intestine" are often used to...
in coeliacs. IRP induced the rapid expression of interleukin 15
Interleukin 15
Interleukin 15 is a cytokine with structural similarity to IL-2. Like IL-2, IL-15 binds to and signals through the IL-2/IL-15 beta chain and the common gamma chain . IL-15 is secreted by mononuclear phagocytes following infection by virus...
(IL15) and other factors. Thus IRP activates the immune system. Studies show that, while in normal individuals the peptide is trimmed over time to produce inactive peptide, in celiacs a 19mer may lose a residue from one end or the other, after prolonged incubation that 50% remains intact.
Intraepithileal lymphocytes and IL15
The release of IL15 is a major factor in coeliac disease as IL15 has been found to attract intraepithelial lymphocytes (IEL) that characterize Marsh grade 1 and 2 coeliac disease. Lymphocytes attracted by IL-15 are composed of markers enriched on natural killer cellNatural killer cell
Natural killer cells are a type of cytotoxic lymphocyte that constitute a major component of the innate immune system. NK cells play a major role in the rejection of tumors and cells infected by viruses...
s versus normal helper T-cells. One hypothesis is that IL-15 induces the highly inflammatory Th1 response that activates T-helper cells (DQ2 restricted gliadin specific) that then orchestrate the destructive response, but the reason why inflammatory cells develop prior to gliadin specific helper cells is not known. The IRP response differs from typical responses that stimulate IL15 release, such as viral infection
Virus
A virus is a small infectious agent that can replicate only inside the living cells of organisms. Viruses infect all types of organisms, from animals and plants to bacteria and archaea...
. In addition, other cytokines such as IL12
Interleukin 12
Interleukin 12 is an interleukin that is naturally produced by dendritic cells, macrophages and human B-lymphoblastoid cells in response to antigenic stimulation.-Gene and structure:...
and IL2
IL2
IL2 or IL-2 may refer to:* Ilyushin Il-2 Shturmovik, a World War II era Soviet ground attack aircraft* IL-2 Sturmovik , a flight simulator game named after Ilyushin Il-2 aircraft...
, which are typically associated with T-helper cell stimulation, are not involved. In these two ways the innate peptide activation of T-cells in celiac disease is strange. IL-15 appears to induce increases in MICA and NKG2D that may increase brush-border cell killing.
In addition, innate immunity to IRP peptide is involved in coeliac disease
Coeliac disease
Coeliac disease , is an autoimmune disorder of the small intestine that occurs in genetically predisposed people of all ages from middle infancy onward...
, dermatitis herpetiformis
Dermatitis herpetiformis
Dermatitis herpetiformis , or Duhring's disease,Freedberg, et al. . Fitzpatrick's Dermatology in General Medicine. . McGraw-Hill. ISBN 0-07-138076-0. is a chronic blistering skin condition, characterised by blisters filled with a watery fluid...
and possibly juvenile diabetes. IRP targets monocyte
Monocyte
Monocytes are a type of white blood cell and are part of the innate immune system of vertebrates including all mammals , birds, reptiles, and fish. Monocytes play multiple roles in immune function...
s and increases the production of IL-15 by an HLA-DQ independent pathway, a subsequent study showed that both this region and the "33mer" could create the same response, in cells from both treated coeliacs and non-coeliacs. However, unlike the non-coeliacs, the treated coeliac cells produce the disease marker nitrite
Nitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
. This indicates that another abnormality in people with coeliac disease that allows stimulation to proceed past the normal healthy state. After extensive study, there is no known genetic association for this that appears to stand out at present, and implicates other environmental factors in the defect.
Infiltrating peptides
Some alpha gliadin have other direct-acting properties. Other gliadin peptides, one in a glutamine rich region and another peptide, "QVLQQSTYQLLQELCCQHLW", bind a chemoattractant receptor, CXCR3. Gliadin binds to, blocks and displaces a factor, I-TAC, that binds this receptor. In the process it recruits more CXCR3 receptor, increases MyD88Myd88
Myeloid differentiation primary response gene is a protein that, in humans, is encoded by the MYD88 gene.-Function:In mice, MyD88 is a universal adapter protein as it is used by all TLRs to activate the transcription factor NF-κB. Mal is necessary to recruit Myd88 to TLR 2 and TLR 4, and MyD88...
and Zonulin
Zonulin
Zonulin is a protein that participates in tight junctions between cells of the wall of the digestive tract. Initially discovered in 2000 as the target of zonula occludens toxin, secreted by cholera pathogen Vibrio cholerae, it has been implicated in the pathogenesis of coeliac disease and diabetes...
expression. The factor it displaces, I-TAC, is a T-cell attractant. This peptide may also be involved in increased risk for type 1 diabetes as zonulin
Zonulin
Zonulin is a protein that participates in tight junctions between cells of the wall of the digestive tract. Initially discovered in 2000 as the target of zonula occludens toxin, secreted by cholera pathogen Vibrio cholerae, it has been implicated in the pathogenesis of coeliac disease and diabetes...
production is also a factor. This triggering of zonulin ultimately results in the degradation of tight junctions allowing large solutes, such as proteolytic resistant gliadin fragments to enter behind the brush border membrane cells.
One study examined the effect of ω-5 gliadin, the primary cause of WD-EIA, and found increased permeability of intestinal cells. Other studies show that IgE reactivity to ω-5 gliadin increases greatly when deamidated or crosslinked to transglutaminase.
HLA Class I restrictions to gliadin
HLA class I restrictions to gliadin are not well characterized. HLA-A2HLA-A2
HLA-A2 is a human leukocyte antigen serotype within HLA-A "A" serotype group. The serotype is determined by the antibody recognition of α2 subset of HLA-A α-chains. For A2, the alpha "A" chain are encoded by the HLA-A*02 allele group and the β-chain are encoded by B2M locus. A2 and A*02 are...
presentation has been investigated.
The HLA-A antigens can mediate apoptosis
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
in autoimmune disease and HLA A*0201 in with the HLA-DQ8
HLA-DQ8
HLA-DQ8 is a human leukocyte antigen serotype within the HLA-DQ serotype group. DQ8 is a split antigen of the DQ3 broad antigen. DQ8 is determined by the antibody recognition of β8 and this generally detects the gene product of DQB1*0302....
haplotypes has been documented. The class I sites were found on the carboxyl end of gliadin at positions 123-131, 144-152, and 172-180. The involvement of class I responses may be minor, since antibodies to transglutaminase correlate with pathogenesis and recognition of extracellular matrix and cell surface transglutaminase can explain the destruction within coeliac disease. This process involves antibody-dependent cellular cytotoxicity
Antibody-dependent cellular cytotoxicity
Antibody-Dependent Cell-Mediated Cytotoxicity is a mechanism of cell-mediated immunity whereby an effector cell of the immune system actively lyses a target cell that has been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune...
. With regard to a receptor called FOS, euphemistically called the "Death Receptor", enterocytes appear to overexpress the recept in coeliac lesions, there is speculation that Class I presentation of glaidin, tTG or other peptides that invokes signalling. The role of class I receptor in cell-mediated programmed cell (enterocyte) death is not known.
MIC
These proteins are called MHC class I polypeptide-related sequence A and B. Discovered by sequence homology analysis these proteins are found on the surface of enterocytes of the small intestine, are believed to play a role in disease. Studies to date have revealed no mutation that would increase risk for MICA.HLA-DQ recognition of gluten
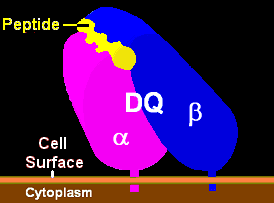 |
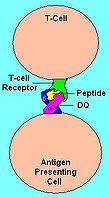 |
| Illustration of HLA-DQ with peptide in the binding pocket | HLA DQ Receptor with bound peptide and TCR |
EWLINE
|
HLA-DQ
HLA-DQ
HLA-DQ is a cell surface receptor type protein found on antigen presenting cells. DQ is an αβ heterodimer of the MHC Class II type. The α and β chains are encoded by HLA-DQA1 and HLA-DQB1, respectively. These two loci are adjacent to each other on chromosome 6p21.3. Both the α-chain and β-chain...
proteins present polypeptide regions of proteins of about 9 amino acids and larger in size (10 to 14 residues in involved in binding is common for gliadin) to T lymphocytes.
Gliadin proteins can be adsorbed by APC. After digestion in the lysozomes of APCs, glaidin peptides can be recycled to the cell's surface bound to DQ, or they can be bound and presented directly from the cell surface. The major source of inflammatory gluten is dietary gluten. Optimal reactivity of gliadin occurs when the protein is partially digested by small intestinal lysozyme
Lysozyme
Lysozyme, also known as muramidase or N-acetylmuramide glycanhydrolase, are glycoside hydrolases, enzymes that damage bacterial cell walls by catalyzing hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in a peptidoglycan and between...
and trypsin
Trypsin
Trypsin is a serine protease found in the digestive system of many vertebrates, where it hydrolyses proteins. Trypsin is produced in the pancreas as the inactive proenzyme trypsinogen. Trypsin cleaves peptide chains mainly at the carboxyl side of the amino acids lysine or arginine, except when...
into proteolytic digests. These polypeptides of gluten can then make their way behind the epithelial layer of cells (membrane), where APCs and T-cells reside in the lamina propria
Lamina propria
The lamina propria is a constituent of the moist linings known as mucous membranes or mucosa, which line various tubes in the body ....
. (See: Underlying conditions)
The APC bearing DQ-gliadin peptide on the surface can bind to T-cells that have an antibody-like T-cell receptor the specifically recognized DQ2.5 with gliadin. The complex (APC-DQ-glaidin) thus stimulates the gliadin specific T-cells to divide. These cells cause B-cells that recognize gliadin to proliferate. The B-cells mature into plasma cell
Plasma cell
Plasma cells, also called plasma B cells, plasmocytes, and effector B cells, are white blood cells which produce large volumes of antibodies. They are transported by the blood plasma and the lymphatic system...
s producing anti-gliadin antibodies
Anti-gliadin antibodies
Antigliadin antibodies are produced in response to gliadin, a prolamin found in wheat. In bread wheat it is encoded by three different alleles, AA, BB, and DD. These alleles can produce slightly different gliadins, which can cause the body to produce different antibodies...
. This does not cause coeliac disease and is an unknown factor in idiopathic disease. Enteropathy is believed to occur when tissue transglutaminase
Tissue transglutaminase
Tissue transglutaminase is an enzyme of the transglutaminase family. Like other transglutaminases, it crosslinks proteins between an ε-amino group of a lysine residue and a γ-carboxamide group of glutamine residue, creating an inter- or intramolecular bond that is highly resistant to proteolysis...
(tTG) covelantly links itself to gliadin
Gliadin
Gliadin is a glycoprotein present in wheat and several other cereals within the grass genus Triticum. Gliadins are prolamins and are separated on the basis of electrophoretic mobility and isoelectric focusing.- Types :...
peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
s that enter the lamina propria
Lamina propria
The lamina propria is a constituent of the moist linings known as mucous membranes or mucosa, which line various tubes in the body ....
of the intestinal villus. The resulting structure can be presented by APC (with the same gliadin recognizing DQ isoforms) to T-cells, and B-cells can produce anti-transglutaminase antibodies
Anti-transglutaminase antibodies
Anti-transglutaminase antibodies are autoantibodies against the transglutaminase protein. Antibodies serve an important role in the immune system by detecting cells and substances that the rest of the immune system then eliminates. These cells and substance can be foreign and also can be producd...
. This appears to result in the destruction of the villi. The release of gliadin by transglutaminase does not lessen disease. When tTG-gliadin undergoes hydrolysis (steals a water to cut the two apart), the result is deamidated gliadin. Deamidated gliadin peptides are more inflammatory relative to natural peptides. Deamidated gliadin is also found in foods that have added gluten, such as wheat bread, food pastes.
The major gluten proteins that are involved in coeliac disease are the α-gliadin isoforms. Alpha gliadin is composed of repeated motifs that, when digested, can be presented by HLA-DQ molecules. DQ2.5 recognizes several motifs in gluten proteins, and therefore HLA-DQ can recognize many motifs on each gliadin (see Understanding DQ haplotypes and DQ isoforms on the right) However, numbers of different proteins from the grass tribe Triticeae
Triticeae
Triticeae is a tribe within the Pooideae subfamily of grasses that includes genera with many domesticated species. Major crop genera are found in this tribe including wheat , barley, and rye; crops in other genera include some for human consumption and others used for animal feed or rangeland...
have been found to carry motifs presented by HLA DQ2.5 and DQ8. Wheat
Wheat
Wheat is a cereal grain, originally from the Levant region of the Near East, but now cultivated worldwide. In 2007 world production of wheat was 607 million tons, making it the third most-produced cereal after maize and rice...
has a large number of these proteins because its genome contains chromosomes derived from two goat grass
Aegilops
Aegilops is a genus of plants generally known as goatgrasses and belonging to the grass family, Poaceae. There are about 23 species and numerous sub species in the genus. Various members of the genus are classed as agricultural weeds. Growing through the winter, they resemble winter wheat...
species and a primitive wheat species. The positions of these motifs in different species, strains and isoforms may vary because of insertions and deletions in sequence. There are a large number of wheat variants, and a large number of gliadin
Gliadin
Gliadin is a glycoprotein present in wheat and several other cereals within the grass genus Triticum. Gliadins are prolamins and are separated on the basis of electrophoretic mobility and isoelectric focusing.- Types :...
s in each variant, and thus many potential sites. These proteins once identified and sequenced can be surveyed by sequence homology searches.

HLA-DQ2.5
HLA-DQ recognition of gliadin is critical to the pathogenesis of gluten-sensitive enteropathy, it also appears to be involved in idiopathic gluten sensitivity (See:Understanding DQ Haplotypes and DQ isoforms on the right).HLA-DQ2
HLA-DQ2
HLA-DQ2 is a serotype group within HLA-DQ serotyping system. The serotype is determined by the antibody recognition of β2 subset of DQ β-chains. The β-chain of DQ is encoded by HLA-DQB1 locus and DQ2 are encoded by the HLA-DQB1*02 allele group. This group currently contains two common alleles,...
primarily presents gliadins with the HLA-DQ isoform DQ2.5 (DQ α5-β2) isoform. DQA1*0202:DQB1*0201 homozygotes (DQ α2-β2) also appear to be able to present pathogenic gliadin peptides, but a smaller set with lower binding affinity.
DQ2.5 and α-gliadin
Many of these gliadin motifs are substrates for tissue transglutaminaseTissue transglutaminase
Tissue transglutaminase is an enzyme of the transglutaminase family. Like other transglutaminases, it crosslinks proteins between an ε-amino group of a lysine residue and a γ-carboxamide group of glutamine residue, creating an inter- or intramolecular bond that is highly resistant to proteolysis...
and therefore can be modified by deamidation in the gut to create more inflammatory peptides. The most important recognition appears to be directed toward the α-/β-gliadin
Gliadin
Gliadin is a glycoprotein present in wheat and several other cereals within the grass genus Triticum. Gliadins are prolamins and are separated on the basis of electrophoretic mobility and isoelectric focusing.- Types :...
s. An example of the repetition of a motif across many proteins, the α-2 gliadin (57-68) and (62-75) are also found on α-4, α-9 gliadin. Many gliadins contain the "α-20 motif", which is found in wheat and other Triticeae genera.(see also: "α-20" gliadin motifs). Alpha-2 secalin
Secalin
Secalin is a protein found in the grain rye.Secalin is one of the forms of gluten that people with coeliac disease cannot tolerate, and thus rye should be avoided by people with this disease. It is generally recommended that such people follow a gluten free diet.In bread making with rye flour, this...
, the glutinous protein in rye, is composed of two amino-terminal overlapping T-cell sites at positions (8-19) and (13-23).

A2-gliadin
Although T-cell responses to many prolamins can be found be found in coeliac disease, one particular gliadin, α2-gliadin appears to be the focus of T-cells. These responses were dependent on prior treatment with tissue transglutaminaseTissue transglutaminase
Tissue transglutaminase is an enzyme of the transglutaminase family. Like other transglutaminases, it crosslinks proteins between an ε-amino group of a lysine residue and a γ-carboxamide group of glutamine residue, creating an inter- or intramolecular bond that is highly resistant to proteolysis...
. Α2-gliadin differs from the other α-gliadins, specifically because it contains an insert of 14 amino acids. This particular insertion creates 6 T-cell sites where, in the most similar gliadins, there are 2 or less sites. The sites belong to three epitope groups "α-I", "α-II", and "α-III" The insertion also creates a larger region of α-gliadin that is resistant to gastrointestinal proteases. The smallest digest of trypsin and chymotrypsin for the region is a 33mer. This particular region has three tissue transglutaminase sites, two sites that lie within the 14 amino acid insertion, a region of maximal stimulation are found with more than 80% reduction in response for native, un-deaminated, sequence at the position. Because of the density of T-cell sites on the "33mer" the affinity for deamidated gliadin is strongly indicates that it may be best treated as a single T-cell site of much higher affinity. This site alone may fulfill all the T-helper cell adaptive immune requirements with HLA-DQ2.5 involvement in some coeliac disease.
DQ2.5 and γ-gliadin
While gamma glaidin is not as important to DQ2.5 mediated disease as α-2 gliadin there are a number of identified motifs.The gamma epitopes identified are DQ2-"γ-I", -"γ-II" (γ30), -"γ-III", -"γ-IV", -"γ-VI" and -"γ-VII"
Some of these epitopes are recognized in children who do not have T-cell reactivities toward α-2 gliadin. A 26 residue proteolytic resistance fragment has been found on γ-5 gliadin, positions 26–51, that has multiple transglutaminase and T-cell epitopes. This site has 5 overlapping T-cells sites of DQ2-"γ-II", -"γ-III", -"γ-IV", and "γ-glia 2". Computer analysis of 156 prolamins and glutelins revealed many more resistant fragments, one , a γ-gliadin, containing 4 epitopes was 68 amino acids in length.
DQ2 and glutelins
Triticeae glutelins presented by DQ2 is some coeliacs. In wheat, the low molecular weight glutenins often share structural similarity with the prolamins of the similar species of Triticeae. Two motifs, K1-like (46-60), pGH3-like (41-59) and GF1(33-51) have been identified. High molecular weight glutenin has also been identified as a potentially toxic protein Some of the HMW glutenins increase response with transglutaminase treatment, indicating the sites might be similar to alpha-gliadin and gamma gliadin T-cell sites.DQ2.2 restricted gliadin sites
DQ2.2 can present a fewer number of lower affinity sites relative to DQ2.5. Some of these sites are found on γ-gliadin the gliadin most similar to prolaminProlamin
Prolamins are a group of plant storage proteins having a high proline content and found in the seeds of cereal grains: wheat , barley , rye , corn , sorghum and as a minor protein, avenin in oats. They are characterised by a high glutamine and proline content and are generally soluble only in...
s of other Triticeae
Triticeae
Triticeae is a tribe within the Pooideae subfamily of grasses that includes genera with many domesticated species. Major crop genera are found in this tribe including wheat , barley, and rye; crops in other genera include some for human consumption and others used for animal feed or rangeland...
genera, a gliadin that appears to similar to ancestral. Antigen presenting cells bearing DQ2.2 can present alpha gliadin sites, for example alpha-II region of the "33mer" and therefore the "33mer" may have a role in DQ2.2 bearing individuals, but the binding capacity is substantially lower.
HLA-DQ8
HLA-DQ8HLA-DQ8
HLA-DQ8 is a human leukocyte antigen serotype within the HLA-DQ serotype group. DQ8 is a split antigen of the DQ3 broad antigen. DQ8 is determined by the antibody recognition of β8 and this generally detects the gene product of DQB1*0302....
confers susceptibility to coeliac disease but in a fashion somewhat similar to DQ2.5. Homozygotes of DQ8, DQ2.5/DQ8 and DQ8/DQ2.2 are higher than expected based on levels in the general population.(see: Understanding DQ haplotypes and DQ isoforms). HLA-DQ8 is generally not as involved in the most severe complications, and it does not recognize the "33mer" of α-2 gliadin to the same degree as DQ2.5. There are a smaller number of gliadin (prolamin) peptides presented by HLA-DQ8. A few studies have been done on the adaptive immune response for DQ8/DQ2- individuals. DQ8 appears to rely much more on adaptive immunity to the carboxyl half of alpha gliadins. In addition, it appear to react with gamma gliadin to a degree comparable to DQ2.5. T-cell responses to the high molecular weight glutenin may be more important with DQ8 mediated than DQ2.5 mediated celiac disease.
Antibody recognition
Antibody recognition of gluten is complex. Direct binding to gluten such as anti-gliadin antibodiesAnti-gliadin antibodies
Antigliadin antibodies are produced in response to gliadin, a prolamin found in wheat. In bread wheat it is encoded by three different alleles, AA, BB, and DD. These alleles can produce slightly different gliadins, which can cause the body to produce different antibodies...
has an ambiguous pathogenesis in coeliac disease. The crosslinking of gliadin with tissue transglutaminase leads to the production of anti-transglutaminase antibodies
Anti-transglutaminase antibodies
Anti-transglutaminase antibodies are autoantibodies against the transglutaminase protein. Antibodies serve an important role in the immune system by detecting cells and substances that the rest of the immune system then eliminates. These cells and substance can be foreign and also can be producd...
, but this is mediated through T-cell recognition of gliadin. The allergic recognition of gliadin
Wheat allergy
Wheat allergy is a food allergy, but can also be a contact allergy resulting from occupational exposure. Like all allergies wheat allergy involves IgE and mast cell response. Typically the allergy is limited to the seed storage proteins of wheat, some reactions are restricted to wheat proteins,...
by mast cells, eosinophiles in the presence of IgE has notable direct consequences, such as exercise-induced anaphylaxis
Exercise-induced anaphylaxis
Exercise-induced anaphylaxis is a syndrome in which the symptoms of anaphylaxis occur related to exercise.In some incidents, individuals experienced anaphylaxis only after combination exposure to a triggering agent and increased physical activity shortly after the ingestion of the triggering agent...
.
Anti-gliadin antibodies, like those detected in celiac disease bind to the α-2 gliadin(57-73).
This site is within the T-cell reactive "33mer" presented by DQ2.5. There has been some suggestion wheat plays a role in juvenile diabetes as antibodies to the non-glutinous seed storage glb-1 (a globulin) are implicated in crossreactive autoantigenic antibodies that destroy islet cells in the pancreas. Anti-gliadin antibodies have been found to synapsin I
Synapsin
The synapsins are a family of proteins that have long been implicated in the regulation of neurotransmitter release at synapses. Specifically, they are thought to be involved in regulating the number of synaptic vesicles available for release via exocytosis at any one time.Current studies suggest...
Omega-gliadin and the HMW Glutenin subunit antibodies have been found most commonly in individuals with exercise-induced anaphylaxis
Exercise-induced anaphylaxis
Exercise-induced anaphylaxis is a syndrome in which the symptoms of anaphylaxis occur related to exercise.In some incidents, individuals experienced anaphylaxis only after combination exposure to a triggering agent and increased physical activity shortly after the ingestion of the triggering agent...
and Baker's allergy, and represent a potent class of gluten allergens. Non-glutinous proteins in wheat are also allergens, these include: LTP (albumin
Albumin
Albumin refers generally to any protein that is water soluble, which is moderately soluble in concentrated salt solutions, and experiences heat denaturation. They are commonly found in blood plasma, and are unique to other blood proteins in that they are not glycosylated...
/globulin
Globulin
Globulin is one of the three types of serum proteins, the others being albumin and fibrinogen. Some globulins are produced in the liver, while others are made by the immune system. The term globulin encompasses a heterogeneous group of proteins with typical high molecular weight, and both...
), thioredoxin
Thioredoxin
Thioredoxin is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes. In humans, it is encoded by the TXN gene. Loss-of-function mutation of either of the two human thioredoxin genes is lethal at the four-cell stage of the...
-hB, and wheat flour peroxidase
Peroxidase
Peroxidases are a large family of enzymes that typically catalyze a reaction of the form:For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides...
. A particular 5 residue peptide, Gln-Gln-Gln-Pro-Pro motif, has been found to be a major wheat allergen.









