Kiliani-Fischer synthesis
Encyclopedia
The Kiliani–Fischer synthesis, named for German
chemists Heinrich Kiliani and Hermann Emil Fischer
, is a method for synthesizing
monosaccharides. It proceeds via synthesis and hydrolysis
of a cyanohydrin
, thus elongating the carbon chain of an aldose
by one carbon atom while preserving stereochemistry
on all the previously present chiral
carbons. The new chiral carbon is produced with both stereochemistries, so the product of a Kiliani–Fischer synthesis is a mixture of two diastereomer
ic sugars, called epimer
s. For example, D-arabinose
is converted to a mixture of D-glucose
and D-mannose
.
and aldonic acid
lactone
intermediates. The first step is to react the starting sugar with aqueous cyanide
(typically NaCN); the cyanide undergoes nucleophilic addition
to the carbonyl
group of the sugar (while sugars tend to exist mainly as cyclic
hemiacetal
, they are always in chemical equilibrium
with their open-chain aldehyde
or ketone
forms, and in the case of these aldoses it is that aldehyde form that reacts in this synthesis). The cyanohydrin
resulting from this addition is heated in water, which hydrolyzes
the cyanide
into a carboxylic acid
group that quickly reacts with itself to form a more stable lactone
. Now there are two diastereomeric lactones in the reaction mixture. They are separated (by chromatography
, partition into different solvents, or any of the numerous other separation
methods) and then the desired lactone is reduced with a sodium amalgam
. As illustrated below, D-arabinose
is converted to a mixture of D-glucononitrile and D-mannononitrile, which is then converted to D-gluconolactone and D-mannonolactone, separated, and reduced to D-glucose
or D-mannose
. The chemical yield by this method tends to be around 30%.
, using palladium
on barium sulfate
as the catalyst and water as the solvent
, to form an imine
. Due to the presence of water, the imine quickly hydrolyzes to form an aldehyde, thus the final sugars are produced in just two steps rather than three. The separation of the isomers is then performed at the stage of the sugar products themselves rather than at the lactone intermediates.
The special catalyst is needed to avoid further reduction of the aldehyde
group to a hydroxyl
group, which would yield an alditol. These catalysts that limit hydrogenation
to one step are called poisoned catalysts; Lindlar palladium is another example.
The reactions below illustrate this improved method for the conversion of L-threose
to L-lyxose
and L-xylose
.
D-glyceraldehyde (1) leads to the tetrose
s D-erythrose (2a) and D-threose (2b). Those lead to the pentose
s D-ribose (3a) and D-arabinose (3b), and D-xylose (3c) and D-lyxose (3d), respectively. The next iteration leads to the hexose
s D-allose (4a) and D-altrose (4b), D-glucose (4c) and D-mannose (4d),
D-gulose (4e) and D-idose (4f), and
D-galactose (4g) and D-talose (4h). The D-heptose
s and beyond are available by continuing the sequence, and enantiomeric L series is available by starting the sequence with L-glyceraldehyde.
In practice, the Kiliani–Fischer synthesis is usually used for production of sugars that are difficult or impossible to obtain from natural sources. While it does provide access to every possible stereoisomer of any desired aldose, the process is limited in by its low yield and use of toxic reagents. In addition, the process requires having a supply of the previous sugar in the series, which may itself require substantial synthetic work if it is not readily available. For example, if successive iterations of the Kiliani–Fischer synthesis are used, the overall yield drops approximately exponentially for each additional iteration.
The process only provides direct access to aldoses, whereas some sugars of interest may instead be ketoses. Some ketoses may be accessible from similar aldoses by isomerization via an enediol intermediate; for example, on standing in aqueous base, glucose
, fructose
, and mannose
will slowly interconvert since they share an enediol form. (See mutarotation
). Some unusual sugars are also accessible via aldol addition.
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
chemists Heinrich Kiliani and Hermann Emil Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
, is a method for synthesizing
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
monosaccharides. It proceeds via synthesis and hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of a cyanohydrin
Cyanohydrin
A cyanohydrin is a functional group found in organic compounds. Cyanohydrins have the formula R2CCN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids...
, thus elongating the carbon chain of an aldose
Aldose
An aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
by one carbon atom while preserving stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
on all the previously present chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
carbons. The new chiral carbon is produced with both stereochemistries, so the product of a Kiliani–Fischer synthesis is a mixture of two diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
ic sugars, called epimer
Epimer
In chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
s. For example, D-arabinose
Arabinose
Arabinose is an aldopentose – a monosaccharide containing five carbon atoms, and including an aldehyde functional group.For biosynthetic reasons, most saccharides are almost always more abundant in nature as the "D"-form, or structurally analogous to D-glyceraldehyde.For sugars, the D/L...
is converted to a mixture of D-glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and D-mannose
Mannose
Mannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. It is not part of human metabolism, but is a component of microbial cell walls, and is therefore a target of the immune system and also of antibiotics....
.
Classical Kiliani–Fischer synthesis
The original version of the Kiliani–Fischer synthesis proceeds through cyanohydrinCyanohydrin
A cyanohydrin is a functional group found in organic compounds. Cyanohydrins have the formula R2CCN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids...
and aldonic acid
Aldonic acid
An aldonic acid is any of a family of sugar acids obtained by oxidation of the aldehyde functional group of an aldose to form a carboxylic acid functional group. Thus, their general chemical formula is HOOC-n-CH2OH...
lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
intermediates. The first step is to react the starting sugar with aqueous cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
(typically NaCN); the cyanide undergoes nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
to the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group of the sugar (while sugars tend to exist mainly as cyclic
Cyclic compound
In chemistry, a cyclic compound is a compound in which a series of atoms is connected to form a loop or ring.While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. ...
hemiacetal
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
, they are always in chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
with their open-chain aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
forms, and in the case of these aldoses it is that aldehyde form that reacts in this synthesis). The cyanohydrin
Cyanohydrin
A cyanohydrin is a functional group found in organic compounds. Cyanohydrins have the formula R2CCN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids...
resulting from this addition is heated in water, which hydrolyzes
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
the cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
into a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
group that quickly reacts with itself to form a more stable lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
. Now there are two diastereomeric lactones in the reaction mixture. They are separated (by chromatography
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
, partition into different solvents, or any of the numerous other separation
Separation process
In chemistry and chemical engineering, a separation process, or simply a separation, is any mass transfer process used to convert a mixture of substances into two or more distinct product mixtures, at least one of which is enriched in one or more of the mixture's constituents. In some cases, a...
methods) and then the desired lactone is reduced with a sodium amalgam
Sodium amalgam
Sodium amalgam, commonly denoted Na, is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions involving mercury as a major component. Sodium amalgam is often used in reactions as strong reducing agents with better handling properties compared...
. As illustrated below, D-arabinose
Arabinose
Arabinose is an aldopentose – a monosaccharide containing five carbon atoms, and including an aldehyde functional group.For biosynthetic reasons, most saccharides are almost always more abundant in nature as the "D"-form, or structurally analogous to D-glyceraldehyde.For sugars, the D/L...
is converted to a mixture of D-glucononitrile and D-mannononitrile, which is then converted to D-gluconolactone and D-mannonolactone, separated, and reduced to D-glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
or D-mannose
Mannose
Mannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. It is not part of human metabolism, but is a component of microbial cell walls, and is therefore a target of the immune system and also of antibiotics....
. The chemical yield by this method tends to be around 30%.
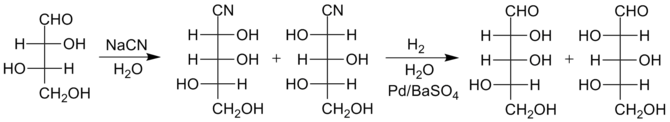
Improved version
More recently, an improved reduction method has been developed that produces somewhat higher yields of the larger sugars. Instead of conversion of the cyanohydrin to a lactone, the cyanohydrin is reduced with hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, using palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
on barium sulfate
Barium sulfate
Barium sulfate is the inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it...
as the catalyst and water as the solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
, to form an imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
. Due to the presence of water, the imine quickly hydrolyzes to form an aldehyde, thus the final sugars are produced in just two steps rather than three. The separation of the isomers is then performed at the stage of the sugar products themselves rather than at the lactone intermediates.
The special catalyst is needed to avoid further reduction of the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
group to a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group, which would yield an alditol. These catalysts that limit hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
to one step are called poisoned catalysts; Lindlar palladium is another example.
The reactions below illustrate this improved method for the conversion of L-threose
Threose
Threose is a four-carbon monosaccharide or carbohydrate with molecular formula C4H8O4. It has a terminal aldehyde group rather than a ketone in its linear chain, and so is considered part of the aldose family of monosaccharides...
to L-lyxose
Lyxose
Lyxose is an aldopentose — a monosaccharide containing five carbon atoms, and including an aldehyde functional group. It has chemical formula 5105.Lyxose occurs only rarely in nature, for example, as a component of bacterial glycolipids.- See also :...
and L-xylose
Xylose
Xylose is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is the precursor to hemicellulose, one of the main constituents of biomass...
.

Uses and limitations
Both enantiomers of glyceraldehyde are commercially available, so one can access any stereoisomer of any chain-length aldose by an appropriate number of repeated applications of the Kiliani–Fischer synthesis. The trioseTriose
A triose is a monosaccharide, or simple sugar, containing three carbon atoms. There are only three possible trioses: L-Glyceraldehyde and D-Glyceraldehyde, both aldotrioses because the carbonyl group is at the end of the chain, and dihydroxyacetone, a ketotriose because the carbonyl group is in...
D-glyceraldehyde (1) leads to the tetrose
Tetrose
A tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde functional group in position 1 or a ketone functional group in position 2 ....
s D-erythrose (2a) and D-threose (2b). Those lead to the pentose
Pentose
A pentose is a monosaccharide with five carbon atoms. Pentoses are organized into two groups. Aldopentoses have an aldehyde functional group at position 1...
s D-ribose (3a) and D-arabinose (3b), and D-xylose (3c) and D-lyxose (3d), respectively. The next iteration leads to the hexose
Hexose
In organic chemistry, a hexose is a monosaccharide with six carbon atoms, having the chemical formula C6H12O6. Hexoses are classified by functional group, with aldohexoses having an aldehyde at position 1, and ketohexoses having a ketone at position 2....
s D-allose (4a) and D-altrose (4b), D-glucose (4c) and D-mannose (4d),
D-gulose (4e) and D-idose (4f), and
D-galactose (4g) and D-talose (4h). The D-heptose
Heptose
A heptose is a monosaccharide with seven carbon atoms.They have either an aldehyde functional group in position 1 or a ketone functional group in position 2 ....
s and beyond are available by continuing the sequence, and enantiomeric L series is available by starting the sequence with L-glyceraldehyde.
In practice, the Kiliani–Fischer synthesis is usually used for production of sugars that are difficult or impossible to obtain from natural sources. While it does provide access to every possible stereoisomer of any desired aldose, the process is limited in by its low yield and use of toxic reagents. In addition, the process requires having a supply of the previous sugar in the series, which may itself require substantial synthetic work if it is not readily available. For example, if successive iterations of the Kiliani–Fischer synthesis are used, the overall yield drops approximately exponentially for each additional iteration.
The process only provides direct access to aldoses, whereas some sugars of interest may instead be ketoses. Some ketoses may be accessible from similar aldoses by isomerization via an enediol intermediate; for example, on standing in aqueous base, glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
, fructose
Fructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
, and mannose
Mannose
Mannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. It is not part of human metabolism, but is a component of microbial cell walls, and is therefore a target of the immune system and also of antibiotics....
will slowly interconvert since they share an enediol form. (See mutarotation
Mutarotation
Mutarotation is the change in the optical rotation that occurs by epimerization...
). Some unusual sugars are also accessible via aldol addition.

