Lanosterol synthase
Encyclopedia
Lanosterol synthase is an oxidosqualene cyclase
(OSC) enzyme
that converts (S)-2,3-oxidosqualene
((S)-2,3-epoxysqualene) to a protosterol cation and finally to lanosterol
. Lanosterol
is a key four-ringed intermediate in cholesterol
biosynthesis. In humans, lanosterol synthase is encoded by the LSS gene
.
In eukaryotes, lanosterol synthase is an integral monotopic protein
associated with the cytosolic side of the endoplasmic reticulum
. Some evidence suggests that the enzyme
is a soluble (non-membrane bound) protein
in the few prokaryotes that produce it.
Due to the enzyme’s role in cholesterol
biosynthesis, there is interest in lanosterol synthase inhibitors
as potential cholesterol reducing drugs, to complement existing statins.
, mutagenesis
studies, and homology modeling
, it is still not fully understood how the enzyme
catalyzes the formation of lanosterol
.
Initial Epoxide Protonation and Ring Opening: Before the acquisition of the protein’s X-ray crystal structure, site-directed mutagenesis
was used to determine residues key to the enzyme’s catalytic activity. It was determined that an aspartic acid
residue (D455) and two histidine
residues (H146 and H234) were essential to enzyme function. Corey et al. hypothesized that the aspartic acid acts by protonating the substrate’s epoxide
ring, thus increasing its susceptibility to intramolecular
attack by the nearest double bond
, with H146 possibly intensifying the proton donor ability of the aspartic acid through hydrogen bonding. After acquisition of the X-ray crystal structure of the enzyme, the role of D455 as a proton donor to the substrate’s epoxide was confirmed, though it was found that D455 is more likely stabilized by hydrogen bonding from two cysteine
residues (C456 and C533) than from the earlier suggested histidine.
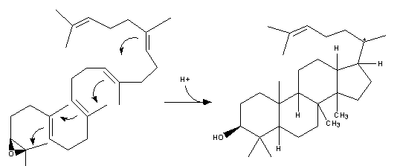
Ring Formation Cascade: Epoxide protonation activates the substrate, setting off a cascade of ring forming reactions. Four rings in total (A through D) are formed, producing the cholesterol
backbone. Though the idea of a concerted formation of all four rings had been entertained in the past, kinetic studies with (S)-2,3-oxidosqualene
analogs showed that product formation is achieved through discrete carbocation
intermediates (see Figure 1). Isolation of monocyclic and bicyclic products from lanosterol synthase mutants has further weakened the hypothesis of a concerted mechanism. Evidence suggests that epoxide ring opening and A ring formation is concerted, though.
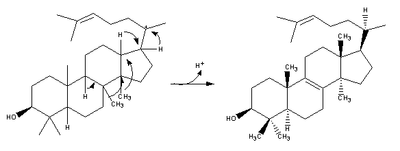
. The enzyme active site
is in the center of the protein, closed off by a constricted channel. Passage of the (S)-2,3-epoxysqualene substrate through the channel requires a change in protein conformation. In eukaryotes, a hydrophobic surface (6% of the total enzyme surface area) is the ER
membrane-binding region (see Figure 2).
The enzyme contains five fingerprint regions containing Gln
-Trp
motifs, which are also present in the highly analogous bacterial enzyme squalene-hopene cyclase. Residues of these fingerprint regions contain stacked sidechains which are thought to contribute to enzyme stability during the highly exergonic
cyclization reactions catalyzed by the enzyme.
, a key four-ringed intermediate in cholesterol
biosynthesis. Thus, it in turn provides the precursor to estrogens, androgens, progesterones, and glucocorticoids. In eukaryotes the enzyme is bound to the cytosolic side of the endoplasmic reticulum
membrane. While cholesterol
synthesis is mostly associated with eukaryotes, few prokaryotes have been found to express lanosterol synthase; it has been found as a soluble protein in Methylococcus capsulatus
.
Catalysis of Epoxylanosterol Formation: Lanosterol synthase also catalyzes the cyclization of 2,3;22,23-diepoxysqualene to 24(S),25-epoxylanosterol, which is later converted to 24(S),25-epoxycholesterol. Since the enzyme affinity for this second substrate is greater than for the monoepoxy (S)-2,3-epoxysqualene, under partial inhibition conversion of 2,3;22,23-diepoxysqualene to 24(S),25-epoxylanosterol is favored over lanosterol
synthesis. This has relevance for disease prevention and treatment (see Disease Relevance, below).
. The widely popular statin
drugs currently used to lower LDL (low-density lipoprotein) cholesterol function by inhibiting HMG-CoA reductase
activity. Because this enzyme catalyzes the formation of precursors far upstream of (S)-2,3-epoxysqualene
and cholesterol, statins may negatively influence amounts of intermediates required for other biosynthetic pathways (e.g. synthesis of isoprenoids, coenzyme Q
). Thus, lanosterol synthase, which is more closely tied to cholesterol biosynthesis than HMG-CoA reductase
, is an attractive drug target.
Lanosterol synthase inhibitors are thought to lower LDL and VLDL cholesterol by a dual control mechanism. Studies in which lanosterol synthase is partially inhibited have shown both a direct decrease in lanosterol
formation and a decrease in HMG-CoA reductase
activity. The oxysterol
24(S),25-epoxylanosterol, which is preferentially formed over lanosterol
during partial lanosterol synthase inhibition, is believed to be responsible for this inhibition of HMG-CoA reductase
activity.
. Phylogenetic trees constructed from the amino acid sequences of OSCs in diverse organisms suggest a single common ancestor, and that the synthesis pathway evolved only once. The discovery of steranes including cholestane
in 2.7-billion year-old shales from Pilbara Craton
, Australia
, suggests that eukaryotes with OSCs and complex steroid machinery were present early in earth’s history.
Cyclase
A cyclase is an enzyme, almost always a lyase, that catalyzes a chemical reaction to form a cyclic compound. Important cyclase enzymes include:* Adenylyl cyclase, which forms cyclic AMP from adenosine triphosphate...
(OSC) enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that converts (S)-2,3-oxidosqualene
2,3-Oxidosqualene
2,3- Oxidosqualene ' is an intermediate in the synthesis of the membrane sterol precursors lanosterol and cycloartenol, as well as saponins. It is formed from squalene by squalene monooxygenase...
((S)-2,3-epoxysqualene) to a protosterol cation and finally to lanosterol
Lanosterol
Lanosterol is a tetracyclic triterpenoid, which is the compound from which all steroids are derived.-Role in creation of steroids:Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields...
. Lanosterol
Lanosterol
Lanosterol is a tetracyclic triterpenoid, which is the compound from which all steroids are derived.-Role in creation of steroids:Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields...
is a key four-ringed intermediate in cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
biosynthesis. In humans, lanosterol synthase is encoded by the LSS gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
.


In eukaryotes, lanosterol synthase is an integral monotopic protein
Integral monotopic protein
Integral monotopic proteins, are permanently attached to the membrane from one side.Three-dimensional structures of the following integral monotopic proteins have been determined:*prostaglandin H2 syntheses 1 and 2...
associated with the cytosolic side of the endoplasmic reticulum
Endoplasmic reticulum
The endoplasmic reticulum is an organelle of cells in eukaryotic organisms that forms an interconnected network of tubules, vesicles, and cisternae...
. Some evidence suggests that the enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
is a soluble (non-membrane bound) protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
in the few prokaryotes that produce it.
Due to the enzyme’s role in cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
biosynthesis, there is interest in lanosterol synthase inhibitors
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
as potential cholesterol reducing drugs, to complement existing statins.
Mechanism
Though some data on the mechanism has been obtained by the use of suicide inhibitorsSuicide inhibition
Suicide inhibition, also known as suicide inactivation or mechanism-based inhibition, is a form of irreversible enzyme inhibition that occurs when an enzyme binds a substrate analogue and forms an irreversible complex with it through a covalent bond during the "normal" catalysis reaction...
, mutagenesis
Site-directed mutagenesis
Site-directed mutagenesis, also called site-specific mutagenesis or oligonucleotide-directed mutagenesis, is a molecular biology technique in which a mutation is created at a defined site in a DNA molecule. In general, this form of mutagenesis requires that the wild type gene sequence be known...
studies, and homology modeling
Homology modeling
Homology modeling, also known as comparative modeling of protein refers to constructing an atomic-resolution model of the "target" protein from its amino acid sequence and an experimental three-dimensional structure of a related homologous protein...
, it is still not fully understood how the enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
catalyzes the formation of lanosterol
Lanosterol
Lanosterol is a tetracyclic triterpenoid, which is the compound from which all steroids are derived.-Role in creation of steroids:Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields...
.
Initial Epoxide Protonation and Ring Opening: Before the acquisition of the protein’s X-ray crystal structure, site-directed mutagenesis
Site-directed mutagenesis
Site-directed mutagenesis, also called site-specific mutagenesis or oligonucleotide-directed mutagenesis, is a molecular biology technique in which a mutation is created at a defined site in a DNA molecule. In general, this form of mutagenesis requires that the wild type gene sequence be known...
was used to determine residues key to the enzyme’s catalytic activity. It was determined that an aspartic acid
Aspartic acid
Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
residue (D455) and two histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
residues (H146 and H234) were essential to enzyme function. Corey et al. hypothesized that the aspartic acid acts by protonating the substrate’s epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
ring, thus increasing its susceptibility to intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
attack by the nearest double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
, with H146 possibly intensifying the proton donor ability of the aspartic acid through hydrogen bonding. After acquisition of the X-ray crystal structure of the enzyme, the role of D455 as a proton donor to the substrate’s epoxide was confirmed, though it was found that D455 is more likely stabilized by hydrogen bonding from two cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residues (C456 and C533) than from the earlier suggested histidine.
Ring Formation Cascade: Epoxide protonation activates the substrate, setting off a cascade of ring forming reactions. Four rings in total (A through D) are formed, producing the cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
backbone. Though the idea of a concerted formation of all four rings had been entertained in the past, kinetic studies with (S)-2,3-oxidosqualene
2,3-Oxidosqualene
2,3- Oxidosqualene ' is an intermediate in the synthesis of the membrane sterol precursors lanosterol and cycloartenol, as well as saponins. It is formed from squalene by squalene monooxygenase...
analogs showed that product formation is achieved through discrete carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
intermediates (see Figure 1). Isolation of monocyclic and bicyclic products from lanosterol synthase mutants has further weakened the hypothesis of a concerted mechanism. Evidence suggests that epoxide ring opening and A ring formation is concerted, though.
Enzyme Structure
Lanosterol synthase is a two-domain monomeric protein composed of two connected (α/α) barrel domains and three smaller β-structuresBeta barrel
A beta barrel is a large beta-sheet that twists and coils to form a closed structure in which the first strand is hydrogen bonded to the last.Beta-strands in beta-barrels are typically arranged in an antiparallel fashion...
. The enzyme active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
is in the center of the protein, closed off by a constricted channel. Passage of the (S)-2,3-epoxysqualene substrate through the channel requires a change in protein conformation. In eukaryotes, a hydrophobic surface (6% of the total enzyme surface area) is the ER
Endoplasmic reticulum
The endoplasmic reticulum is an organelle of cells in eukaryotic organisms that forms an interconnected network of tubules, vesicles, and cisternae...
membrane-binding region (see Figure 2).
The enzyme contains five fingerprint regions containing Gln
GLN
GLN or GLn may refer to:* Glutamine, the most abundant naturally occurring amino acid in the human body* Global Location Number, a thirteen digit number used to identify parties and physical locations...
-Trp
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
motifs, which are also present in the highly analogous bacterial enzyme squalene-hopene cyclase. Residues of these fingerprint regions contain stacked sidechains which are thought to contribute to enzyme stability during the highly exergonic
Exergonic reaction
An exergonic reaction is a chemical reaction where the change in the Gibbs free energy is negative, indicating a spontaneous reaction. Symbolically, the release of Gibbs free energy, G, in an exergonic reaction is denoted as...
cyclization reactions catalyzed by the enzyme.
Biological Function
Catalysis of Lanosterol Formation: Lanosterol synthase catalyzes the conversion of (S)-2,3-epoxysqualene to lanosterolLanosterol
Lanosterol is a tetracyclic triterpenoid, which is the compound from which all steroids are derived.-Role in creation of steroids:Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields...
, a key four-ringed intermediate in cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
biosynthesis. Thus, it in turn provides the precursor to estrogens, androgens, progesterones, and glucocorticoids. In eukaryotes the enzyme is bound to the cytosolic side of the endoplasmic reticulum
Endoplasmic reticulum
The endoplasmic reticulum is an organelle of cells in eukaryotic organisms that forms an interconnected network of tubules, vesicles, and cisternae...
membrane. While cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
synthesis is mostly associated with eukaryotes, few prokaryotes have been found to express lanosterol synthase; it has been found as a soluble protein in Methylococcus capsulatus
Methylococcus capsulatus
Methylococcus capsulatus is an obligately methanotrophic gram-negative, non-motile coccoid bacterium. M. capsulatus cells are encapsulated and tend to have a diplococcoid arrangement. In addition to methane, M. capsulatus is able to oxidize some organic hydrogen containing compounds such as...
.
Catalysis of Epoxylanosterol Formation: Lanosterol synthase also catalyzes the cyclization of 2,3;22,23-diepoxysqualene to 24(S),25-epoxylanosterol, which is later converted to 24(S),25-epoxycholesterol. Since the enzyme affinity for this second substrate is greater than for the monoepoxy (S)-2,3-epoxysqualene, under partial inhibition conversion of 2,3;22,23-diepoxysqualene to 24(S),25-epoxylanosterol is favored over lanosterol
Lanosterol
Lanosterol is a tetracyclic triterpenoid, which is the compound from which all steroids are derived.-Role in creation of steroids:Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields...
synthesis. This has relevance for disease prevention and treatment (see Disease Relevance, below).
Disease Relevance
Enzyme Inhibitors as Cholesterol Lowering Drugs: Interest has grown in lanosterol synthase inhibitors as drugs to lower blood cholesterol and treat atherosclerosisAtherosclerosis
Atherosclerosis is a condition in which an artery wall thickens as a result of the accumulation of fatty materials such as cholesterol...
. The widely popular statin
Statin
Statins are a class of drugs used to lower cholesterol levels by inhibiting the enzyme HMG-CoA reductase, which plays a central role in the production of cholesterol in the liver. Increased cholesterol levels have been associated with cardiovascular diseases, and statins are therefore used in the...
drugs currently used to lower LDL (low-density lipoprotein) cholesterol function by inhibiting HMG-CoA reductase
HMG-CoA reductase
HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids...
activity. Because this enzyme catalyzes the formation of precursors far upstream of (S)-2,3-epoxysqualene
2,3-Oxidosqualene
2,3- Oxidosqualene ' is an intermediate in the synthesis of the membrane sterol precursors lanosterol and cycloartenol, as well as saponins. It is formed from squalene by squalene monooxygenase...
and cholesterol, statins may negatively influence amounts of intermediates required for other biosynthetic pathways (e.g. synthesis of isoprenoids, coenzyme Q
Coenzyme Q
Coenzyme Q10, also known as ubiquinone, ubidecarenone, coenzyme Q, and abbreviated at times to CoQ10 , CoQ, Q10, or Q, is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the number of isoprenyl chemical subunits in its tail.This oil-soluble, vitamin-like substance...
). Thus, lanosterol synthase, which is more closely tied to cholesterol biosynthesis than HMG-CoA reductase
HMG-CoA reductase
HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids...
, is an attractive drug target.
Lanosterol synthase inhibitors are thought to lower LDL and VLDL cholesterol by a dual control mechanism. Studies in which lanosterol synthase is partially inhibited have shown both a direct decrease in lanosterol
Lanosterol
Lanosterol is a tetracyclic triterpenoid, which is the compound from which all steroids are derived.-Role in creation of steroids:Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields...
formation and a decrease in HMG-CoA reductase
HMG-CoA reductase
HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids...
activity. The oxysterol
Oxysterol
Oxysterols are oxidized derivatives of cholesterol, which are important in many biological processes, including cholesterol homeostasis, sphingolipid metabolism, platelet aggregation, apoptosis, and protein prenylation.Some examples of oxysterols include:*...
24(S),25-epoxylanosterol, which is preferentially formed over lanosterol
Lanosterol
Lanosterol is a tetracyclic triterpenoid, which is the compound from which all steroids are derived.-Role in creation of steroids:Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields...
during partial lanosterol synthase inhibition, is believed to be responsible for this inhibition of HMG-CoA reductase
HMG-CoA reductase
HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids...
activity.
Evolution
It is believed that oxidosqualene cyclases (OSCs, the class to which lanosterol cyclase belongs) evolved from bacterial squalene-hopene cyclase (SHC), which is involved with the formation of hopanoidsHopanoids
Hopanoids are natural pentacyclic compounds based on the chemical structure of hopane. Their primary function is to improve plasma membrane strength and rigidity in bacteria. In eukaryotes cholesterol serves a similar function...
. Phylogenetic trees constructed from the amino acid sequences of OSCs in diverse organisms suggest a single common ancestor, and that the synthesis pathway evolved only once. The discovery of steranes including cholestane
Cholestane
Cholestane is a saturated 27-carbon steroid precursor which serves as the basis for many organic molecules.- Derivatives of cholestane :Derivatives are classified in two families:* Sterols * Cholestenes...
in 2.7-billion year-old shales from Pilbara Craton
Pilbara craton
The Pilbara craton , along with the Kaapvaal craton are the only remaining areas of pristine Archaean 3.6-2.7 Ga crust on Earth...
, Australia
Australia
Australia , officially the Commonwealth of Australia, is a country in the Southern Hemisphere comprising the mainland of the Australian continent, the island of Tasmania, and numerous smaller islands in the Indian and Pacific Oceans. It is the world's sixth-largest country by total area...
, suggests that eukaryotes with OSCs and complex steroid machinery were present early in earth’s history.

