
Mass spectrum analysis
Encyclopedia
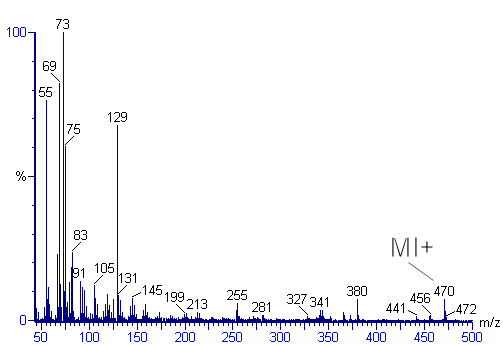
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
. Organic chemists obtain mass spectra
Mass spectrum
A mass spectrum is an intensity vs. m/z plot representing a chemical analysis. Hence, the mass spectrum of a sample is a pattern representing the distribution of ions by mass in a sample. It is a histogram usually acquired using an instrument called a mass spectrometer...
of chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s as part of structure elucidation and the analysis is part of every organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
curriculum.
Basic peaks
Mass spectra have several distinct sets of peaks:- the molecular ion
- isotope peaks
- fragmentation peaks
- metastable peaks
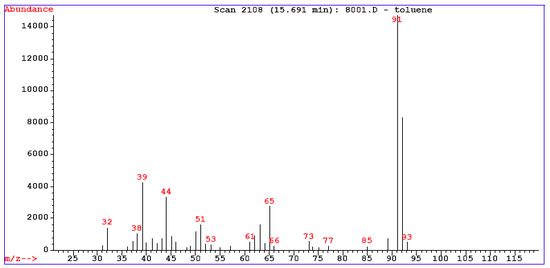
Mass spectra first of all display the molecular ion (or parent ion) peak which is a radical cation M+. as a result of removing one electron from the molecule. In the spectrum for toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
for example the molecular ion peak is located at 92 m/e corresponding to its molecular mass
Molecular mass
The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u...
. The molecular ion peak does not always appear or can be weak. The height of the molecular ion peak diminishes with branching and with increasing mass in a homologous series
Homologous series
In chemistry, a homologous series is a series of compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group, and showing a gradation in physical properties as a result of increase in molecular size and mass...
.
Identifying the molecular ion can be difficult. A useful aid is the nitrogen rule: if the mass is an even number, the compound contains no nitrogen or an even number of nitrogens. Molecular ion peaks are also often preceded by a M-1 or M-2 peak resulting from loss of a hydrogen radical or dihydrogen.
More peaks are visible with m/e ratios larger than the molecular ion peak due to isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
distributions. The value of 92 in the toluene example corresponds to the monoisotopic mass
Monoisotopic mass
The monoisotopic mass is the sum of the masses of the atoms in a molecule using the unbound, ground-state, rest mass of the principal isotope for each element instead of the isotopic average mass. For typical organic compounds, where the monoisotopic mass is most commonly used, this also results...
of a molecule of toluene entirely composed of the most abundant isotopes (1H and 12C). The so-called M+1 peak corresponds to a fraction of the molecules with one higher isotope incorporated (2H or 13C) and the M+2 peak has two higher isotopes. The natural abundance
Natural abundance
In chemistry, natural abundance refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass of these isotopes is the atomic weight listed for the element in the periodic table...
of the higher isotopes is low for frequently encountered elements such as hydrogen, carbon and nitrogen and the intensity of isotope peaks subsequently low and the intensity quickly diminishes with total mass. In halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s on the other hand higher isotopes have a large abundance which results in a specific mass signature for halogen containing compounds.
Peaks with mass less than the molecular ion are the result of fragmentation of the molecule. These peaks are called daughter peaks. The peak with the highest ratio is called the base peak which is not necessarily the molecular ion. Many reaction pathways exist for fragmentation but only newly formed cations will show up in the mass spectrum and not radical fragments or neutral fragments.
Metastable peaks are broad peaks at non-integer mass values. These peaks result from molecular fragments with lower kinetic energy because of fragmentations taking place ahead of the ionization chamber.
Fragmentation
The fragmentation pattern not only allows the determination of the mass of an unknown compound but also allows guessing the molecular structure especially in combination with the calculation of the degree of unsaturationDegree of unsaturation
The degree of unsaturation formula is used in organic chemistry to help draw chemical structures. The formula lets the user determine how many rings, double bonds, and triple bonds are present in the compound to be drawn...
from the molecular formula (when available). Neutral fragments frequently lost are carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
, water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, and hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
.
fragmentations arise from:
- homolysisHomolysisIn general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
processes. An example is the cleavage of carbon-carbon bondCarbon-carbon bondA carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is said to be formed between one hybridized orbital from each...
s next to a heteroatomHeteroatomIn organic chemistry, a heteroatom is any atom that is not carbon or hydrogen. Usually, the term is used to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure...

- In this depiction single-electron movements are indicated by a single-headed arrow.
- Rearrangement reactionRearrangement reactionA rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
s, for example a retro Diels-Alder reactionDiels-Alder reactionThe Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
extruding neutral ethylene:
- Rearrangement reaction

- or the McLafferty rearrangementMcLafferty rearrangementThe McLafferty rearrangement is a reaction observed in mass spectrometry. It is sometimes found that a molecule containing a keto-group undergoes β-cleavage, with the gain of the γ-hydrogen atom...
. As it is not always obvious where a lone electron resides in a radical cation a square bracket notation is often used.
Some general rules:
- Cleavage occurs at alkyl substituted carbons reflecting the order generally observed in carbocationCarbocationA carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s. - Double bonds and arene fragments tend to resist fragmentation.
- Allylic cations are stable and resist fragmentation.
- the even-electron rule stipulates that even-electron species (cations but not radical ions) will not fragment into two odd-electron species but rather to another cation and a neutral molecule.
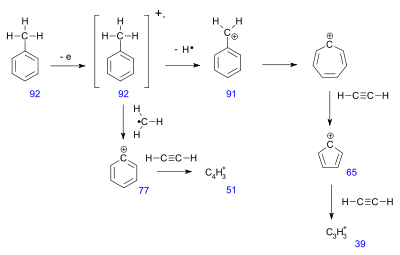
Toluene example
The mass spectrum for tolueneToluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
has around 30 signals. Several peaks can be rationalized in this fragmentation pattern.
Isotope effects
Isotope peaks within a spectrum can help in structure elucidation. Compounds containing halogens (especially chlorineChlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
and bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
) produce very distinct isotope peaks. The mass spectrum of methylbromide has two prominent peaks of equal intensity at 94 (M) and 96 (M+2) and then two more at 79 and 81 belonging to the bromine fragment.
Even when compounds only contain elements with less intense isotope peaks (carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
or oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
), the distribution of these peaks can be used to assign the spectrum to the correct compound. For example, two compounds with identical mass of 150, C8H12N3+ and C9H10O2+, will have two different M+2 intensities which makes it possible to distinguish between them.
Natural abundance of some elements
The next table gives the isotope distributions for some elements. Some elements like phosphorusPhosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
and fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
only exist as a single isotope, with a natural abundance of 100%.
| Isotope | % nat. abundance !! atomic mass | |
|---|---|---|
| 1H | 99.985 | 1.007825 |
| 2H | 0.015 | 2.0140 |
| 12C | 98.89 | 12 (definition) |
| 13C Carbon-13 Carbon-13 is a natural, stable isotope of carbon and one of the environmental isotopes. It makes up about 1.1% of all natural carbon on Earth.- Detection by mass spectrometry :... |
1.11 | 13.00335 |
| 14N | 99.64 | 14.00307 |
| 15N | 0.36 | 15.00011 |
| 16O | 99.76 | 15.99491 |
| 17O | 0.04 | |
| 18O | 0.2 | 17.99916 |
| 28Si | 92.23 | 27.97693 |
| 29Si | 4.67 | 28.97649 |
| 30Si | 3.10 | 29.97376 |
| 32S | 95.0 | 31.97207 |
| 33S | 0.76 | 32.97146 |
| 34S | 4.22 | 33.96786 |
| 37Cl | 24.23 | |
| 35Cl | 75.77 | 34.96885 |
| 79Br | 50.69 | 78.9183 |
| 81Br | 49.31 | 80.9163 |
See also
- COmponent Detection AlgorithmCOmponent Detection AlgorithmThe COmponent Detection Algorithm is a name for a type of LC-MS and chemometrics software algorithm focused on detecting peaks in noisy chromatograms often obtained using the electospray ionization technique....
(CODA), an algorithm used in mass spectrometry data analysis

