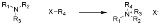
Menshutkin reaction
Encyclopedia
The Menshutkin reaction in organic chemistry
converts a tertiary amine to a quaternary ammonium salt by reaction with an alkyl halide:
The reaction has been named after its discoverer, the Russia
n chemist
Nikolai Menshutkin
, who described the procedure in 1890. Depending on the source, his name (and the reaction named after him) is spelled as Menšutkin, Menshutkin or Menschutkin.
The reaction between amine
s and alkyl halogenides is hard to control, making alternative routes (for instance reductive amination
) more attractive. However, when quaternary ammonium salts are the desired end product, this reaction becomes an interesting option. Yields are good and the reaction is easily performed. R1-R4 can but do not have to be identical. Some phase transfer catalysts (PTC)
can be prepared according to the Menshutkin reaction, for instance the synthesis of triethyl benzyl ammonium chloride (TEBA) from triethylamine
and benzyl chloride
:
 Reactions speed up with polar aprotic solvents and higher reaction temperatures. Leaving group
Reactions speed up with polar aprotic solvents and higher reaction temperatures. Leaving group
s facilitate the reaction in the order chlorine
< bromine
< iodine
.
system the reaction rate is not only accelerated (150000 fold compared to quinuclidine
) but the halide order is also changed:
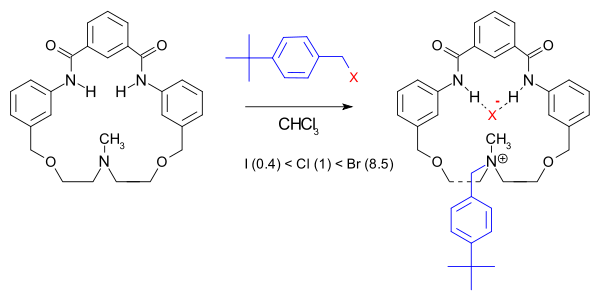 The rate-acceleration is attributed to increased transition state
The rate-acceleration is attributed to increased transition state
stabilization due to hydrogen bonding in the macrocyclic pocket.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
converts a tertiary amine to a quaternary ammonium salt by reaction with an alkyl halide:
The reaction has been named after its discoverer, the Russia
Russia
Russia or , officially known as both Russia and the Russian Federation , is a country in northern Eurasia. It is a federal semi-presidential republic, comprising 83 federal subjects...
n chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
Nikolai Menshutkin
Nikolai Menshutkin
Nikolai Aleksandrovich Menshutkin was a Russian chemist who discovered the process of converting a tertiary amine to a quaternary ammonium salt via the reaction with an alkyl halide, now known as the Menshutkin reaction.-Biography:...
, who described the procedure in 1890. Depending on the source, his name (and the reaction named after him) is spelled as Menšutkin, Menshutkin or Menschutkin.
The reaction between amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s and alkyl halogenides is hard to control, making alternative routes (for instance reductive amination
Reductive amination
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine...
) more attractive. However, when quaternary ammonium salts are the desired end product, this reaction becomes an interesting option. Yields are good and the reaction is easily performed. R1-R4 can but do not have to be identical. Some phase transfer catalysts (PTC)
Phase transfer catalyst
In chemistry, a phase transfer catalyst or PTC is a catalyst that facilitates the migration of a reactant from one phase into another phase where reaction occurs. Phase transfer catalysis is a special form of heterogeneous catalysis. Ionic reactants are often soluble in an aqueous phase but...
can be prepared according to the Menshutkin reaction, for instance the synthesis of triethyl benzyl ammonium chloride (TEBA) from triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
and benzyl chloride
Benzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colourless liquid is a reactive organochlorine compound that is a widely used chemical building block.-Preparation:...
:

Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
s facilitate the reaction in the order chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
< bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
< iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
.
Scope
In one particular macrocycleMacrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
system the reaction rate is not only accelerated (150000 fold compared to quinuclidine
Quinuclidine
Quinuclidine is an organic compound and a bicyclic amine and used as a catalyst and a chemical building block. It is a strong base with pKa of the conjugate acid of 11.0. This is due to greater availability of the nitrogen lone pair...
) but the halide order is also changed:

Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
stabilization due to hydrogen bonding in the macrocyclic pocket.

