Methoxymethylenetriphenylphosphine
Encyclopedia
Methoxymethylenetriphenylphosphine is a Wittig reagent with used as an reagent
in the homologization of aldehyde
s and ketone
s to extended aldehydes, an organic reaction
first reported in 1958 .
The reagent can be prepared (scheme 1) by reaction of triphenylphosphine
1 with chloromethyl methyl ether
2 in diethyl ether
to the phosphonium salt
3. 3 can also be prepared from triphenylphosphine, methylal and acetyl chloride
, a reaction avoiding the costly and carcinogenic chloroalkyl ether and especially useful for large batches.
This salt is deprotonated
to the phosphonium ylide Methoxymethylenetriphenylphosphine 4 by phenyllithium
.
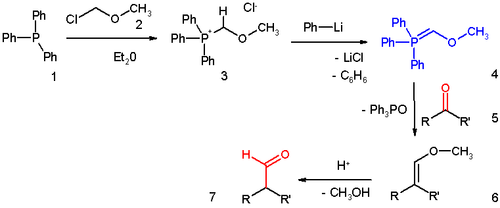 This reagent reacts with a ketone or aldehyde 5 in a Wittig reaction
This reagent reacts with a ketone or aldehyde 5 in a Wittig reaction
to the enol ether
6, which can be converted to the aldehyde 7 by the application of an acid
.
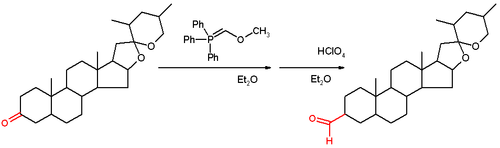
The reaction has been applied to the steroid
tigogenone (scheme 2) and in the Wender Taxol total synthesis
and in the Stork quinine total synthesis.
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
in the homologization of aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s and ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s to extended aldehydes, an organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
first reported in 1958 .
The reagent can be prepared (scheme 1) by reaction of triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
1 with chloromethyl methyl ether
Chloromethyl methyl ether
Chloroalkyl ethers are a class of organic compounds with the general structure R-O-n-Cl, characterized as an ether connected to a chloromethyl group via a alkane chain.Chloromethyl methyl ether is an ether with the formula...
2 in diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
to the phosphonium salt
Phosphonium salt
A phosphonium salt is a salt containing the phosphonium ion such as phosphonium iodide . More commonly, phosphonium refers to a quaternary organic derivative such as tetraphenylphosphonium chloride, 4P+ Cl- and tetramethylphosphonium iodide, [P4]+I−.Alkyltriphenylphosphonium salts are widely...
3. 3 can also be prepared from triphenylphosphine, methylal and acetyl chloride
Acetyl chloride
Acetyl chloride, CH3COCl, also known as ethanoyl chloride or acyl chloride, is an acid chloride derived from acetic acid. It belongs to the class of organic compounds called acyl halides. It is a colorless liquid. Acetyl chloride does not exist in nature, because contact with water would hydrolyze...
, a reaction avoiding the costly and carcinogenic chloroalkyl ether and especially useful for large batches.
This salt is deprotonated
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
to the phosphonium ylide Methoxymethylenetriphenylphosphine 4 by phenyllithium
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
.

Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
to the enol ether
Enol ether
An enol ether is an alkene with an alkoxy substituent. The general structure is R_1R_2C=CR_3-O-R_4 with R an alkyl or an aryl group. Enol ethers and enamines are so-called activated alkenes or electron rich alkenes because the oxygen atom donates electrons to the double bond by forming a resonance...
6, which can be converted to the aldehyde 7 by the application of an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
.
The reaction has been applied to the steroid
Steroid
A steroid is a type of organic compound that contains a characteristic arrangement of four cycloalkane rings that are joined to each other. Examples of steroids include the dietary fat cholesterol, the sex hormones estradiol and testosterone, and the anti-inflammatory drug dexamethasone.The core...
tigogenone (scheme 2) and in the Wender Taxol total synthesis
Wender Taxol total synthesis
The Wender Taxol total synthesis in organic chemistry describes a Taxol total synthesis by the group of Paul A. Wender at Stanford University published in 1997. This synthesis has much in common with the Holton Taxol total synthesis in that it is a linear synthesis starting from a naturally...
and in the Stork quinine total synthesis.


