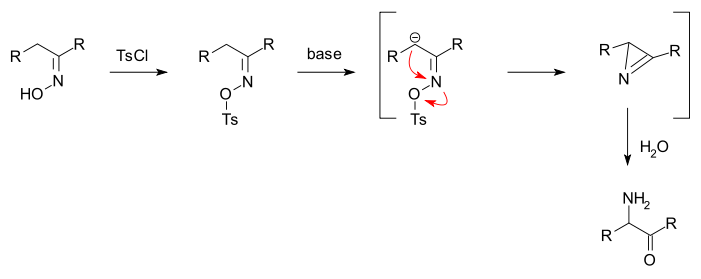
Neber rearrangement
Encyclopedia
The Neber rearrangement is an organic reaction
in which an oxime
is converted into an alpha-aminoketone in a rearrangement reaction
.
The oxime is first converted to a ketoxime tosylate by reaction with tosyl chloride. Added base
forms a carbanion
which displaces the tosylate group in a nucleophilic displacement to a azirine and added water subsequently hydrolyses it to the aminoketone.
The Beckmann rearrangement
is a side reaction.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which an oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
is converted into an alpha-aminoketone in a rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
.
The oxime is first converted to a ketoxime tosylate by reaction with tosyl chloride. Added base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
forms a carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
which displaces the tosylate group in a nucleophilic displacement to a azirine and added water subsequently hydrolyses it to the aminoketone.
The Beckmann rearrangement
Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann , is an acid-catalyzed rearrangement of an oxime to an amide...
is a side reaction.