
Nickel-cadmium battery
Encyclopedia

Rechargeable battery
A rechargeable battery or storage battery is a group of one or more electrochemical cells. They are known as secondary cells because their electrochemical reactions are electrically reversible. Rechargeable batteries come in many different shapes and sizes, ranging anything from a button cell to...
using nickel oxide hydroxide and metallic cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
as electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s.
The abbreviation NiCad is a registered trademark of SAFT Corporation, although this brand name is commonly used
Genericized trademark
A genericized trademark is a trademark or brand name that has become the colloquial or generic description for, or synonymous with, a general class of product or service, rather than as an indicator of source or affiliation as intended by the trademark's holder...
to describe all Ni–Cd batteries. The abbreviation NiCd is derived from the chemical symbol
Chemical symbol
A chemical symbol is a 1- or 2-letter internationally agreed code for a chemical element, usually derived from the name of the element, often in Latin. Only the first letter is capitalised...
s of nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
(Ni) and cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
(Cd).
There are two types of Ni–Cd batteries: sealed and vented. This article mainly deals with sealed cells.
Applications
Sealed Ni–Cd cells may be used individually, or assembled into battery packs containing two or more cells. Small cells are used for portable electronicsElectronics
Electronics is the branch of science, engineering and technology that deals with electrical circuits involving active electrical components such as vacuum tubes, transistors, diodes and integrated circuits, and associated passive interconnection technologies...
and toy
Toy
A toy is any object that can be used for play. Toys are associated commonly with children and pets. Playing with toys is often thought to be an enjoyable means of training the young for life in human society. Different materials are used to make toys enjoyable and cuddly to both young and old...
s, often using cells manufactured in the same sizes as primary cell
Primary cell
A primary cell is any kind of battery in which the electrochemical reaction is not reversible, rendering the cell non-rechargeable. A common example of a primary cell is the disposable battery. Unlike a secondary cell, the reaction cannot be reversed by running a current into the cell; the chemical...
s. When Ni–Cd batteries are substituted for primary cells, the lower terminal voltage and smaller ampere-hour capacity may reduce performance as compared to primary cells. Miniature button cells are sometimes used in photographic equipment, hand-held lamps (flashlight or torch), computer-memory standby, toys, and novelties.
Specialty Ni–Cd batteries are used in cordless and wireless telephones, emergency lighting, and other applications. With a relatively low internal resistance
Internal resistance
A practical electrical power source which is a linear electric circuit may, according to Thévenin's theorem, be represented as an ideal voltage source in series with an impedance. This resistance is termed the internal resistance of the source. When the power source delivers current, the measured...
, they can supply high surge currents. This makes them a favourable choice for remote-controlled electric model airplanes, boats, and cars, as well as cordless power tools and camera flash units.
Larger flooded cells are used for aircraft starting batteries, electric vehicles, and standby power
Uninterruptible power supply
An uninterruptible power supply, also uninterruptible power source, UPS or battery/flywheel backup, is an electrical apparatus that provides emergency power to a load when the input power source, typically mains power, fails...
.
Voltage
Ni–Cd cells have a nominal cell potential of 1.2 volts (V). This is lower than the 1.5 V of alkaline and zinc–carbon primary cells, and consequently they are not appropriate as a replacement in all applications. However, the 1.5 V of a primary alkaline cell refers to its initial, rather than average, voltage. Unlike alkaline and zinc–carbon primary cells, a Ni–Cd cell's terminal voltage only changes a little as it discharges. Because many electronic devices are designed to work with primary cells that may discharge to as low as 0.90 to 1.0 V per cell, the relatively steady 1.2 V of a Ni–Cd cell is enough to allow operation. Some would consider the near-constant voltage a drawback as it makes it difficult to detect when the battery charge is low.Ni–Cd batteries used to replace 9 V batteries usually only have six cells, for a terminal voltage of 7.2 volts. While most pocket radios will operate satisfactorily at this voltage, some manufacturers such as Varta
VARTA
VARTA AG was a company based in Germany manufacturing batteries for global automotive, industrial and consumer markets. A sales slogan was "you're smarter to fit Varta!" in the mid 1990s...
made 8.4 volt batteries with seven cells for more critical applications.
12 V Ni–Cd batteries are made up of 10 cells connected in series.
History
The first Ni–Cd battery was created by Waldemar JungnerWaldemar Jungner
Ernst Waldemar Jungner, June 19, 1869- August 30, 1924, was a Swedish inventor and engineer. In 1899 he invented the nickel-iron electric storage battery and invented the nickel-cadmium battery.-Literature:...
of Sweden
Sweden
Sweden , officially the Kingdom of Sweden , is a Nordic country on the Scandinavian Peninsula in Northern Europe. Sweden borders with Norway and Finland and is connected to Denmark by a bridge-tunnel across the Öresund....
in 1899. At that time, the only direct competitor was the lead–acid battery, which was less physically and chemically robust. With minor improvements to the first prototypes, energy density rapidly increased to about half of that of primary batteries, and significantly greater than lead–acid batteries. Jungner experimented with substituting iron for the cadmium in varying quantities, but found the iron formulations to be wanting. Jungner's work was largely unknown in the United States. Thomas Edison adapted the battery design where he introduced the nickel–iron battery to the US two years after Jungner had built one. In 1906, Jungner established a factory close to Oskarshamn, Sweden to produce flooded design Ni–Cd batteries.
Production in the United States
The first production in the United StatesUnited States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
began in 1946. Up to this point, the batteries were "pocket type," constructed of nickel-plated steel pockets containing nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
active materials. Around the middle of the twentieth century, sintered
Sintering
Sintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
-plate Ni–Cd batteries became increasingly popular. Fusing nickel powder at a temperature well below its melting point using high pressures creates sintered plates. The plates thus formed are highly porous, about 80 percent by volume. Positive and negative plates are produced by soaking the nickel plates in nickel- and cadmium-active materials, respectively. Sintered plates are usually much thinner than the pocket type, resulting in greater surface area per volume and higher currents. In general, the greater amount of reactive material surface area in a battery, the lower its internal resistance
Internal resistance
A practical electrical power source which is a linear electric circuit may, according to Thévenin's theorem, be represented as an ideal voltage source in series with an impedance. This resistance is termed the internal resistance of the source. When the power source delivers current, the measured...
.
Recent developments
In the past few decades, Ni–Cd batteries have had internal resistance as low as alkaline batteries. Today, all consumer Ni–Cd batteries use the "swiss rollSwiss roll
A Swiss roll or jelly roll is a type of sponge cake roll. The thin cake is made of eggs, flour and sugar and baked in a very shallow rectangular baking tray, called a sheet pan. The cake is removed from the pan and spread with jam or buttercream, rolled up, and served in circular slices.The...
" or "jelly-roll" configuration. This design incorporates several layers of positive and negative material rolled into a cylindrical shape. This design reduces internal resistance as there is a greater amount of electrode in contact with the active material in each cell.
Popularity
Advances in battery-manufacturing technologies throughout the second half of the twentieth century have made batteries increasingly cheaper to produce. Battery-powered devices in general have increased in popularity. As of 2000, about 1.5 billion1000000000 (number)
1,000,000,000 is the natural number following 999,999,999 and preceding 1,000,000,001.In scientific notation, it is written as 109....
Ni–Cd batteries were produced annually.
Up until the mid 1990s, Ni–Cd batteries had an overwhelming majority of the market share for rechargeable batteries in consumer electronics.
Ni–Cd batteries account for 8% of all portable secondary (rechargeable) battery sales in the EU, and in the UK for 9.2% and in Switzerland for 1.3% of all portable battery sales.
Comparison with other batteries
Recently, nickel–metal hydride and lithium-ion batteries have become commercially available and cheaper, the former type now rivaling Ni–Cd batteries in cost. Where energy density is important, Ni–Cd batteries are now at a disadvantage compared with nickel–metal hydride and lithium-ion batteries. However, the Ni–Cd battery is still very useful in applications requiring very high discharge rates because it can endure such discharge with no damage or loss of capacity.Advantages
When compared to other forms of rechargeable battery, the Ni–Cd battery has a number of distinct advantages:- The batteries are more difficult to damage than other batteries, tolerating deep discharge for long periods. In fact, Ni–Cd batteries in long-term storage are typically stored fully discharged. This is in contrast, for example, to lithium ion batteriesLithium ion batteryA lithium-ion battery is a family of rechargeable battery types in which lithium ions move from the negative electrode to the positive electrode during discharge, and back when charging. Chemistry, performance, cost, and safety characteristics vary across LIB types...
, which are less stable and will be permanently damaged if discharged below a minimum voltage. - Ni–Cd batteries typically last longer, in terms of number of charge/discharge cycles, than other rechargeable batteries such as lead/acid batteries.
- Compared to lead–acid batteries, Ni–Cd batteries have a much higher energy densityEnergy densityEnergy density is a term used for the amount of energy stored in a given system or region of space per unit volume. Often only the useful or extractable energy is quantified, which is to say that chemically inaccessible energy such as rest mass energy is ignored...
. A Ni–Cd battery is smaller and lighter than a comparable lead–acid battery. In cases where size and weight are important considerations (for example, aircraft), Ni–Cd batteries are preferred over the cheaper lead–acid batteries. - In consumer applications, Ni–Cd batteries compete directly with alkaline batteriesAlkaline batteryAlkaline batteries are a type of primary batteries dependent upon the reaction between zinc and manganese dioxide . A rechargeable alkaline battery allows reuse of specially designed cells....
. A Ni–Cd cell has a lower capacity than that of an equivalent alkaline cell, and costs more. However, since the alkaline battery's chemical reaction is not reversible, a reusable Ni–Cd battery has a significantly longer total lifetime. There have been attempts to create rechargeable alkaline batteriesRechargeable alkaline batteryRechargeable alkaline battery is a type of alkaline battery that is rechargeable. The first generation rechargeable alkaline technology was developed by Battery Technologies Inc in Canada and licensed to Pure Energy, EnviroCell, Rayovac, and Grandcell...
, or specialized battery chargers for charging single-use alkaline batteries, but none that has seen wide usage. - The terminal voltage of a Ni–Cd battery declines more slowly as it is discharged, compared with carbon–zinc batteries. Since an alkaline battery's voltage drops significantly as the charge drops, most consumer applications are well equipped to deal with the slightly lower Ni–Cd cell voltage with no noticeable loss of performance.
- The capacity of a Ni–Cd battery is not significantly affected by very high discharge currents. Even with discharge rates as high as 50C, a Ni–Cd battery will provide very nearly its rated capacity. By contrast, a lead acid battery will only provide approximately half its rated capacity when discharged at a relatively modest 1.5C.
- Nickel–metal hydrideHydrideIn chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
(NiMHNIMHNIMH or NiMH may refer to:*Nickel-metal hydride battery, a type of rechargeable battery*National Institute of Mental Health, a part of the United States National Institutes of Health...
) batteries are the newest, and most similar, competitor to Ni–Cd batteries. Compared to Ni–Cd batteries, NiMH batteries have a higher capacity and are less toxic, and are now more cost effective. However, a Ni–Cd battery has a lower self-dischargeSelf-dischargeSelf-discharge is a phenomenon in batteries in which internal chemical reactions reduce the stored charge of the battery without any connection between the electrodes...
rate (for example, 20% per month for a Ni–Cd battery, versus 30% per month for a traditional NiMH under identical conditions), although low self-discharge NiMH batteriesLow self-discharge NiMH batteryThe low self-discharge nickel-metal hydride battery was introduced in November 2005. These batteries were developed by Sanyo, who called them "eneloop". Subsequently, other manufacturers also offered LSD NiMH....
are now available, which have substantially lower self-discharge than either Ni–Cd or traditional NiMH batteries. This results in a preference for Ni–Cd over NiMH batteries in applications where the current draw on the battery is lower than the battery's own self-discharge rate (for example, television remote controls). In both types of cell, the self-discharge rate is highest for a full charge state and drops off somewhat for lower charge states. Finally, a similarly sized Ni–Cd battery has a slightly lower internal resistance, and thus can achieve a higher maximum discharge rate (which can be important for applications such as power tools).
Disadvantages
- The primary trade-off with Ni–Cd batteries is their higher cost and the use of cadmium. This heavy metal is an environmental hazard, and is highly toxic to all higher forms of life. They are also more costly than lead–acid batteries because nickel and cadmium cost more.
- One biggest disadvantages is that the battery exhibits a very marked negative temperature coefficient. This means that as the cell temperature rises, the internal resistance falls. This can pose considerable charging problems, particularly with the relatively simple charging systems employed for lead–acid type batteries. Whilst lead–acid batteries can be charged by simply connecting a dynamoDynamo- Engineering :* Dynamo, a magnetic device originally used as an electric generator* Dynamo theory, a theory relating to magnetic fields of celestial bodies* Solar dynamo, the physical process that generates the Sun's magnetic field- Software :...
to them, with a simple electromagnetic cut-out system for when the dynamo is stationary or an over-current occurs, the Ni–Cd battery under a similar charging scheme would exhibit thermal runaway, where the charging current would continue to rise until the over-current cut-out operated or the battery destroyed itself. This is the principal factor that prevents its use as engine-starting batteries. Today with alternator-based charging systems with solid-state regulators, the construction of a suitable charging system would be relatively simple, but the car manufacturers are reluctant to abandon tried-and-tested technology.
See also Nickel–cadmium battery#Problems with Ni–Cd batteries.
Availability
Ni–Cd cells are available in the same sizes as alkaline batteries, from AAA through D, as well as several multi-cell sizes, including the equivalent of a 9 volt battery. A fully charged single Ni–Cd cell, under no load, carries a potential difference of between 1.25 and 1.35 volts, which stays relatively constant as the battery is discharged. Since an alkaline battery near fully discharged may see its voltage drop to as low as 0.9 volts, Ni–Cd cells and alkaline cells are typically interchangeable for most applications.In addition to single cells, batteries exist that contain up to 300 cells (nominally 360 volts, actual voltage under no load between 380 and 420 volts). This many cells are mostly used in automotive and heavy-duty industrial applications. For portable applications, the number of cells is normally below 18 cells (24V). Industrial-sized flooded batteries are available with capacities ranging from 12.5Ah up to several hundred Ah.
Characteristics
The maximum discharge rate for a Ni–Cd battery varies by size. For a common AA-sizeAA battery
An AA battery is a standard size of battery. Batteries of this size are the most commonly used type of in portable electronic devices. An AA battery is composed of a single electrochemical cell...
cell, the maximum discharge rate is approximately 18 amps; for a D size
D battery
A D battery is a size of dry cell. A D cell is cylindrical with electrical contacts at each end; the positive end having a nub or bump...
battery the discharge rate can be as high as 35 amps.
Model-aircraft or -boat builders often take much larger currents of up to a hundred amps or so from specially constructed Ni–Cd batteries, which are used to drive main motors. 5–6 minutes of model operation is easily achievable from quite small batteries, so a reasonably high power-to-weight figure is achieved, comparable to internal combustion motors, though of lesser duration. In this, however, they have been largely superseded by lithium polymer (Lipo) and lithium iron phosphate (LiFe) batteries, which can provide even higher energy densities.
Charging
Ni–Cd batteries can be charged at several different rates, depending on how the cell was manufactured. The charge rate is measured based on the percentage of the amp-hour capacity the battery is fed as a steady current over the duration of the charge. Regardless of the charge speed, more energy must be supplied to the battery than its actual capacity, to account for energy loss during charging, with faster charges being more efficient. For example, an "overnight" charge, might consist of supplying a current equals to one tenth the amperehour rating ( C/10 ) for 14–16 hours; that is, a 100 mAh battery takes 10mA for 14 hours, for a total of 140 mAh to charge at this rate. At the rapid-charge rate, done at 100% of the rated capacity of the battery in 1 hour (1C), the battery holds roughly 80% of the charge, so a 100 mAh battery takes 120 mAh to charge (that is, approximately 1 hour and fifteen minutes). Some specialized batteries can be charged in as little as 10–15 minutes at a 4C or 6C charge rate, but this is very uncommon. It also exponentially increases the risk of the cells overheating and venting due to an internal overpressure condition: the cell's rate of temperature rise is governed by its internal resistance and the square of the charging rate. At a 4C rate, the amount of heat generated in the cell is sixteen times higher than the heat at the 1C rate. The downside to faster charging is the higher risk of overchargingOvercharging
Overcharging can refer to:*Overcharging , a prosecutorial practice*Charging a battery too much*Charging a customer too much...
, which can damage the battery. and the increased temperatures the cell has to endure (which potentially shortens its life).
The safe temperature range when in use is between −20°C and 45°C. During charging, the battery temperature typically stays low, around 0°C (the charging reaction absorbs heat), but as the battery nears full charge the temperature will rise to 45–50°C. Some battery chargers detect this temperature increase to cut off charging and prevent over-charging.
When not under load or charge, a Ni–Cd battery will self-discharge approximately 10% per month at 20°C, ranging up to 20% per month at higher temperatures. It is possible to perform a trickle charge at current levels just high enough to offset this discharge rate; to keep a battery fully charged. However, if the battery is going to be stored unused for a long period of time, it should be discharged down to at most 40% of capacity (some manufacturers recommend fully discharging and even short-circuiting once fully discharged), and stored in a cool, dry environment.
Charge condition
High quality Ni–Cd batteries have a thermal cut-off so if the battery gets too hot the charger stops. If a battery is still warm from discharging and been put on charge, it will not get the full charge possible. In that case, let the battery cool to room temperature, then charge. Watch for the correct polarity. Leave charger in a cool place when charging to get best results.Charging method
A Ni–Cd battery requires a charger with a slightly different voltageVoltage
Voltage, otherwise known as electrical potential difference or electric tension is the difference in electric potential between two points — or the difference in electric potential energy per unit charge between two points...
than for a lead–acid battery, especially if the battery has 11 or 12 cells. Also a charge termination method is needed if a fast charger is used. Often battery pack
Battery pack
A battery pack is a set of any number of identical batteries or individual battery cells. They may be configured in a series, parallel or a mixture of both to deliver the desired voltage, capacity, or power density...
s have a thermal
Thermal
A thermal column is a column of rising air in the lower altitudes of the Earth's atmosphere. Thermals are created by the uneven heating of the Earth's surface from solar radiation, and are an example of convection. The sun warms the ground, which in turn warms the air directly above it...
cut-off
Cut-off
A cut-off, also known as a kutte or "battle jacket" in heavy metal subcultures, is a type of vest or jacket which originated in the biker subculture and has now found popularity in the punk and various heavy metal subcultures...
inside that feeds back to the charger telling it to stop the charging once the battery has heated up and/or a voltage peaking sensing circuit. At room temperature during normal charge conditions the cell voltage increases from an initial 1.2 V to an end-point of about 1.45 V. The rate of rise increases markedly as the cell approaches full charge. The end-point voltage decreases slightly with increasing temperature.
Electrochemistry
A fully charged Ni–Cd cell contains:- a nickel(III) oxide-hydroxide positive electrode platePlate electrodeA plate is a type of electrode that formed part of a vacuum tube. The plate is impressed with a positive charge so that it may capture and flow electrons within a circuit....
. - a cadmiumCadmiumCadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
negative electrode plate. - a separatorSeparatorSeparator can refer to:* Cream separator, separates cream from milk* Community separator, a term of urban planning* Separator , of an oil production plant* Vapor-liquid separator, separates a vapor-liquid mixture...
. - and an alkaline electrolyteElectrolyteIn chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
(potassium hydroxidePotassium hydroxidePotassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
).
Ni–Cd batteries usually have a metal case with a sealing plate equipped with a self-sealing safety valve
Safety valve
A safety valve is a valve mechanism for the automatic release of a substance from a boiler, pressure vessel, or other system when the pressure or temperature exceeds preset limits....
. The positive and negative electrode plates, isolated from each other by the separator, are rolled in a spiral shape inside the case. This is known as the jelly-roll design and allows a Ni–Cd cell to deliver a much higher maximum current than an equivalent size alkaline cell. Alkaline cells have a bobbin construction where the cell casing is filled with electrolyte and contains a graphite rod which acts as the positive electrode. As a relatively small area of the electrode is in contact with the electrolyte (as opposed to the jelly-roll design), the internal resistance for an equivalent sized alkaline cell is higher which limits the maximum current that can be delivered.
The chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s during discharge are:

at the cadmium electrode, and
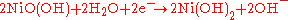
at the nickel electrode. The net reaction during discharge is

During recharge, the reactions go from right to left. The alkaline electrolyte (commonly KOH) is not consumed in this reaction and therefore its Specific Gravity, unlike in lead–acid batteries, is not a guide to its state of charge.
When Jungner built the first Ni–Cd batteries, he used nickel oxide in the positive electrode, and iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
and cadmium materials in the negative. It was not until later that pure cadmium metal and nickel hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
were used. Until about 1960, the chemical reaction was not completely understood. There were several speculations as to the reaction products. The debate was finally resolved by spectrometry, which revealed cadmium hydroxide and nickel hydroxide.
Another historically important variation on the basic Ni–Cd cell is the addition of lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
hydroxide to the potassium hydroxide electrolyte. This was believed to prolong the service life by making the cell more resistant to electrical abuse. The Ni–Cd battery in its modern form is extremely resistant to electrical abuse anyway, so this practice has been discontinued.
Overcharging
OverchargingOvercharging
Overcharging can refer to:*Overcharging , a prosecutorial practice*Charging a battery too much*Charging a customer too much...
must be considered in the design of most rechargeable batteries. In the case of Ni–Cd batteries, there are two possible results of overcharging:
- If the negative electrode is overcharged, hydrogenHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
gas is produced. - If the positive electrode is overcharged, oxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
gas is produced.
For this reason, the negative electrode is always designed for a higher capacity than the positive, to avoid releasing hydrogen gas. There is still the problem of eliminating oxygen gas, to avoid rupture of the cell casing. Ni–Cd cells are vented, with seals
Seal (mechanical)
A mechanical seal is a device which helps join systems or mechanisms together by preventing leakage , containing pressure, or excluding contamination...
that fail at high internal gas pressures. The sealing mechanism must allow gas to escape from inside the cell, and seal again properly when the gas is expelled. This complex mechanism, unnecessary in alkaline batteries, contributes to their higher cost.
Ni–Cd cells dealt with in this article are of the sealed
Seal (mechanical)
A mechanical seal is a device which helps join systems or mechanisms together by preventing leakage , containing pressure, or excluding contamination...
type (see also vented type). Cells of this type consist of a pressure vessel that is supposed to contain any generation of oxygen and hydrogen gasses until they can recombine back to water. Such generation typically occurs during rapid charge and discharge and exceedingly at overcharge condition. If the pressure exceeds the limit of the safety valve, water in the form of gas is lost. Since the vessel is designed to contain an exact amount of electrolyte this loss will rapidly affect the capacity of the cell and its ability to receive and deliver current. To detect all conditions of overcharge demands great sophistication from the charging circuit and a cheap charger will eventually damage even the best quality cells.
Cell reversal
Another potential problem is reverse charging. This can occur due to an error by the user, or more commonly, when a battery of several cells is fully discharged. Because there is a slight variation in the capacity of cells in a battery, one of the cells will usually be fully discharged before the others, at which point reverse charging begins seriously damaging that cell, reducing battery life. The by-product of reverse charging is hydrogen gas, which can be dangerous. Some commentators advise that one should never discharge multi-cell Ni–Cd batteries to zero voltage; for example, incandescent lights should be turned off when they are yellow; before they go out completely.A common form of this deprecation
Deprecation
In the process of authoring computer software, its standards or documentation, deprecation is a status applied to software features to indicate that they should be avoided, typically because they have been superseded...
occurs when cells connected in series develop unequal voltages and discharge near zero voltage. The first cell that reaches zero is pushed beyond to negative voltage and gases generated open the seal and dry the cell.
In modern cells, an excess of anti-polar material (basically active material ballast
Electrical ballast
An electrical ballast is a device intended to limit the amount of current in an electric circuit. A familiar and widely used example is the inductive ballast used in fluorescent lamps, to limit the current through the tube, which would otherwise rise to destructive levels due to the tube's...
at positive electrode) is inserted to allow for moderate negative charge without damage to the cell. This excess material slows down the start of oxygen generation at the negative plate. This means a cell can survive a negative voltage of about −0.2 to −0.4 volts. However if discharge is continued even further, this excess ballast is used up and both electrodes change polarity, causing destructive gassing (gas generation).
Battery pack
Battery pack
A battery pack is a set of any number of identical batteries or individual battery cells. They may be configured in a series, parallel or a mixture of both to deliver the desired voltage, capacity, or power density...
s with multiple cells in series should be operated well above 1 volt per cell to avoid placing the lowest capacity cell in danger of going negative. Battery packs that can be disassembled into cells should be periodically zeroed and charged individually to equalize the voltages. However, this does not help if old and new cells are mixed, since their different capacities will result in different discharge times and voltages.
Memory and lazy battery effects
Ni–Cd batteries may suffer from a "memory effectMemory effect
Memory effect, also known as battery effect, lazy battery effect or battery memory, is an alleged effect observed in nickel cadmium rechargeable batteries that causes them to hold less charge...
" if they are discharged and recharged to the same state of charge
State of charge
State of charge is the equivalent of a fuel gauge for the battery pack in a battery electric vehicle , hybrid vehicle , or plug-in hybrid electric vehicle...
hundreds of times. The apparent symptom is that the battery "remembers" the point in its charge cycle where recharging began and during subsequent use suffers a sudden drop in voltage at that point, as if the battery had been discharged. The capacity of the battery is not actually reduced substantially. Some electronics designed to be powered by Ni–Cd batteries are able to withstand this reduced voltage long enough for the voltage to return to normal. However, if the device is unable to operate through this period of decreased voltage, it will be unable to get enough energy out of the battery, and for all practical purposes, the battery appears "dead" earlier than normal.
There is much evidence that the memory effect story originated from orbiting satellites, where they were typically charging for twelve hours out of twenty-four for several years. After this time, it was found that the capacities of the batteries had declined significantly, but were still perfectly fit for use. It is unlikely that this precise repetitive charging (e.g., 1000 charges / discharges with less than 2% variability) could ever be reproduced by consumers using electrical goods.
An effect with similar symptoms to the memory effect is the so-called voltage depression or lazy battery effect. This results from repeated overcharging; the symptom is that the battery appears to be fully charged but discharges quickly after only a brief period of operation. In rare cases, much of the lost capacity can be recovered by a few deep-discharge cycles, a function often provided by automatic battery chargers. However, this process may reduce the shelf life of the battery. If treated well, a Ni–Cd battery can last for 1000 cycles or more before its capacity drops below half its original capacity.
Dendritic shorting
When not used regularly, dendritesDendrite (metal)
A dendrite in metallurgy is a characteristic tree-like structure of crystals growing as molten metal freezes, the shape produced by faster growth along energetically favourable crystallographic directions. This dendritic growth has large consequences in regards to material properties.Dendrites form...
tend to develop. Dendrites are thin, conductive crystals that may penetrate the separator membrane between electrodes. This leads to internal short circuits and premature failure, long before the 800–1000 charge/discharge cycle life claimed by most vendors. Sometimes, applying a brief, high-current charging pulse to individual cells can clear these dendrites, but they will typically reform within a few days or even hours. Cells in this state have reached the end of their useful life and should be replaced. Many battery guides, circulating on the Internet and online auctions, promise to restore dead cells using the above principle, but achieve very short-term results at best.
Environmental consequences of cadmium
Ni–Cd batteries contain between 6% (for industrial batteries) and 18% (for consumer batteries) cadmiumCadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
, which is a toxic heavy metal
Heavy metals
A heavy metal is a member of a loosely-defined subset of elements that exhibit metallic properties. It mainly includes the transition metals, some metalloids, lanthanides, and actinides. Many different definitions have been proposed—some based on density, some on atomic number or atomic weight,...
and therefore requires special care during battery disposal. In the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
, part of the battery price is a fee for its proper disposal at the end of its service lifetime. Under the so-called "batteries directive" (2006/66/EC), the sale of consumer Ni–Cd batteries has now been banned within the European Union except for medical use; alarm systems; emergency lighting; and portable power tools. This last category is to be reviewed after 4 years. Under the same EU directive, used industrial Ni–Cd batteries must be collected by their producers in order to be recycled in dedicated facilities.
Cadmium, being a heavy metal, can cause substantial pollution
Pollution
Pollution is the introduction of contaminants into a natural environment that causes instability, disorder, harm or discomfort to the ecosystem i.e. physical systems or living organisms. Pollution can take the form of chemical substances or energy, such as noise, heat or light...
when landfill
Landfill
A landfill site , is a site for the disposal of waste materials by burial and is the oldest form of waste treatment...
ed or incinerated
Incineration
Incineration is a waste treatment process that involves the combustion of organic substances contained in waste materials. Incineration and other high temperature waste treatment systems are described as "thermal treatment". Incineration of waste materials converts the waste into ash, flue gas, and...
. Because of this, many countries now operate recycling
Battery recycling
Battery recycling is a recycling activity that aims to reduce the number of batteries being disposed as municipal solid waste. Batteries contain a number of heavy metals and toxic chemicals, their dumping has raised concern over risks of soil contamination and water pollution.-Battery recycling by...
programs to capture and reprocess old batteries.
Safety
Manufacturers typically supply instructions for safe handling, use, and disposal. These warn against physical damage, short-circuiting when fully charged, and overcharging.See also
- Nickel–cadmium battery vented cell type
- Nickel–iron battery
- Battery recyclingBattery recyclingBattery recycling is a recycling activity that aims to reduce the number of batteries being disposed as municipal solid waste. Batteries contain a number of heavy metals and toxic chemicals, their dumping has raised concern over risks of soil contamination and water pollution.-Battery recycling by...
- Battery holderBattery holderA battery holder is one or more compartments or chambers for holding a battery. For dry cells, the holder must also make electrical contact with the battery terminals...
- Nickel metal hydride batteryNickel metal hydride batteryA nickel–metal hydride cell, abbreviated NiMH, is a type of rechargeable battery similar to the nickel–cadmium cell. The NiMH battery uses a hydrogen-absorbing alloy for the negative electrode instead of cadmium. As in NiCd cells, the positive electrode is nickel oxyhydroxide...
- Power-to-weight ratioPower-to-weight ratioPower-to-weight ratio is a calculation commonly applied to engines and mobile power sources to enable the comparison of one unit or design to another. Power-to-weight ratio is a measurement of actual performance of any engine or power sources...

