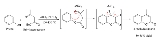
Pechmann condensation
Encyclopedia
The Pechmann condensation is a synthesis of coumarin
s, starting from a phenol
and a carboxylic acid
or ester
containing a β-carbonyl
group . The condensation is performed under acidic conditions. The mechanism involves an esterification/transesterification followed by attack of the activated carbonyl ortho to the oxygen to generate the new ring. The final step is a dehydration, as seen following an aldol condensation
. It was discovered by the German
chemist
Hans von Pechmann
.
With simple phenols, the conditions are harsh, although yields may still be good .
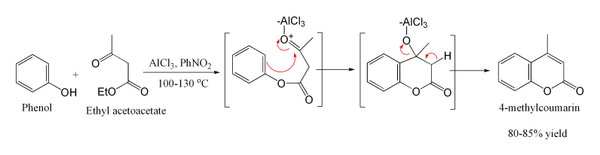 With highly activated phenols such as resorcinol
With highly activated phenols such as resorcinol
, the reaction can be performed under much milder conditions. This provides a useful route to umbelliferone
derivatives:
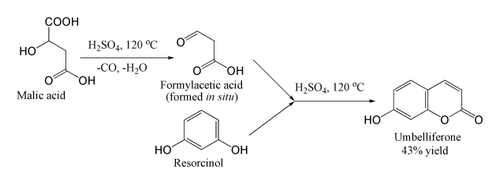
 For coumarins unsubstituted at the 4-position, the method requires the use of formylacetic acid or ester. These are unstable and not commercially available, but the acid may be produced in situ from malic acid
For coumarins unsubstituted at the 4-position, the method requires the use of formylacetic acid or ester. These are unstable and not commercially available, but the acid may be produced in situ from malic acid
and sulfuric acid
above 100°C. As soon as it forms, the formylacetic acid performs the Pechmann condensation. In the example shown, umbelliferone itself is produced, albeit in low yield:
yields a chromone
. This reaction is called Simonis chromone cyclization . The ketone in the ketoester is activated by P2O5 for reaction with the phenol hydroxyl group first, the ester group in it is then activated for electrophilic attack of the arene.
Coumarin
Coumarin is a fragrant chemical compound in the benzopyrone chemical class, found in many plants, notably in high concentration in the tonka bean , vanilla grass , sweet woodruff , mullein , sweet grass , cassia cinnamon and sweet clover...
s, starting from a phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
and a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
or ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
containing a β-carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group . The condensation is performed under acidic conditions. The mechanism involves an esterification/transesterification followed by attack of the activated carbonyl ortho to the oxygen to generate the new ring. The final step is a dehydration, as seen following an aldol condensation
Aldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
. It was discovered by the German
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
Hans von Pechmann
Hans von Pechmann
Hans von Pechmann was a German chemist, renowned for his discovery of diazomethane in 1894. , Pechmann condensation and Pechmann pyrazole synthesis...
.
With simple phenols, the conditions are harsh, although yields may still be good .

Resorcinol
Resorcinol is a dihydroxy benzene. It is the 1,3-isomer of benzenediol with the formula C6H42.-Nomenclature:Benzene-1,3-diol is the name recommended by the International Union of Pure and Applied Chemistry in its 1993 Recommendations for the Nomenclature of Organic Chemistry.-Production:It is...
, the reaction can be performed under much milder conditions. This provides a useful route to umbelliferone
Umbelliferone
Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and beta-umbelliferone, is a widespread natural product of the coumarin family. It occurs in many familiar plants from the Apiaceae family such as carrot, coriander and garden angelica, as well plants from other families such...
derivatives:

Malic acid
Malic acid is an organic compound with the formula HO2CCH2CHOHCO2H. It is a dicarboxylic acid which is made by all living organisms, contributes to the pleasantly sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms , though only the L-isomer exists...
and sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
above 100°C. As soon as it forms, the formylacetic acid performs the Pechmann condensation. In the example shown, umbelliferone itself is produced, albeit in low yield:

Simonis chromone cyclization
In a variation the reaction of phenols and beta-ketoesters and phosphorus pentoxidePhosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
yields a chromone
Chromone
Chromone is a derivative of benzopyran with a substituted keto group on the pyran ring. It is an isomer of coumarin.Derivatives of chromone are collectively known as chromones...
. This reaction is called Simonis chromone cyclization . The ketone in the ketoester is activated by P2O5 for reaction with the phenol hydroxyl group first, the ester group in it is then activated for electrophilic attack of the arene.

