
Pseudoproline
Encyclopedia
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by some chemical or physical process. In the past it was also used to mean a compound that can be imagined to arise from another compound, if one atom is replaced with another atom or group of atoms, but modern...
are artificially created dipeptide
Dipeptide
A dipeptide is a molecule consisting of two amino acids joined by a single peptide bond.Dipeptides are produced from polypeptides by the action of the hydrolase enzyme dipeptidyl peptidase. Dietary proteins are digested to dipeptides and amino acids, and the dipeptides are absorbed more rapidly...
s to minimize aggregation during FMOC solid-phase synthesis
Solid-phase synthesis
In chemistry, solid-phase synthesis is a method in which molecules are bound on a bead and synthesized step-by-step in a reactant solution; compared with normal synthesis in a liquid state, it is easier to remove excess reactant or byproduct from the product. In this method, building blocks are...
of peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
s.
Introduction
The chemical synthesis of large peptides is still limited by problems of low solvationSolvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
during solid phase peptide synthesis (SPPS) or limited solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
of fully protected peptide fragments: even chemoselective ligation
Chemical ligation
Chemical ligation is a set of techniques used for creating long peptide or protein chains. It is the second step of a convergent approach. First, smaller peptides containing 30-50 amino acids are prepared by conventional chemical peptide synthesis. Then, they are completely deprotected...
methods are hampered by self-association of unprotected peptide blocks. The elucidation of the relationship between preferred conformation of a growing peptide chain and its physicochemical properties reveals that β-sheet (beta-sheet) formation is often paralleled by significant decrease in solvation
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
and solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
. Besides attempts to increase the solvation
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
of peptides by external factors, few attempts, i.e. N-substituted Hmb amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
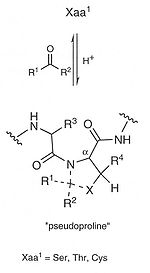
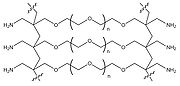
derivatives and pseudoprolines (see figure on the top right) have been reported to modify the intrinsic properties of peptides responsible for aggregation
Particle aggregation
Particle aggregation in materials science is direct mutual attraction between particles via van der Waals forces or chemical bonding....
and secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
formation. Pseudoprolines consist of serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
- (Oxa) or threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
-derived oxazolidines [Oxa(5-Me)] and Cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
-derived thiazolidines (THz) with Proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
-like ring structure (see top right). Mutter and cooworkers have defined oxa- and thiaproline derivatives of serine, threonine, and cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
with Ser(ψPro). Thr(ψPro), and Cys(ψPro), respectively, where the abbreviation ψPro indicates the relationship to proline (with heteroatomic ring substitution in position 4). Pseudoprolines with substitution in position 2 of the proline ring are named Ser/Thr/Cys-(ψR1,R2 Pro). Due to the preference for a cis-amide bond with the preceding residue of C2-substituted pseudoprolines, their incorporation results in a kink conformation of the peptide backbone, thus preventing peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
aggregation, self-association, or β-structure formation.
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
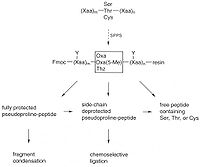
synthesis and in subsequent chain assembly. Pseudoprolines are obtained by reacting the free amino acids with aldehydes or ketone. The coupling of amino acid derivatives to a growing peptide chain containing N-terminal pseudoproline generally results in low yields because of the sterically hindered nature of the oxazolidine (thiazolidine) ring system and the decreased nucleophilicity of the nitrogen atom. Consequently, the preformation of suitably protected dipeptide derivatives of the type FMOC-Xaa1-Oxa/THz-OH is preferable for use in peptide synthesis. Two conceptually different approaches are feasible for preparing oxazolidine- and thiazolidine-ring-containing dipeptide derivatives: (1) the in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
acylation of Ser- or Thr-derived oxazolidines or Cys-derived thiazolidines using acid fluorides or N-carboxyanhydrides (NCA); and (2) the direct insertion of the oxazolidine systems into dipeptides (post-insertion) containing C-terminal Ser or Thr. The method of choice strongly depends on the nature of the pseudoproline as welI as on the substituents at C2 of the cyclic system.
Advantages
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
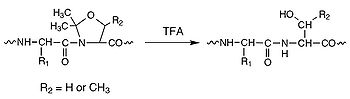
derivatives. The routine use of pseudoproline (oxazolidine
Oxazolidine
Oxazolidine is a five-membered ring compound consisting of three carbons, a nitrogen, a hydrogen, and an oxygen. The oxygen and NH are the 1 and 3 positions, respectively. In oxazolidine derivatives, there is always a carbon between the oxygen and the nitrogen . All of the carbons in oxazolidines...
) dipeptides in the FMOC solid phase pepdide sysnthesis (SPPS) of serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
- and threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
-containing peptides leads to remarkable improvements in quality and yield of crude products and helps avoid unnecessary repeat synthesis of failed sequences. Pseudoproline dipeptides have proven particularly effective in the synthesis of intractable peptides, long peptides/small proteins, and cyclic peptides, enabling in many cases the production of peptides that otherwise could not be made. These dipeptides are extremely easy to use: simply substitute a serine or threonine residue together with the preceding amino acid residue in the peptide sequence with the appropriate pseudoproline dipeptide (see the figure on your right). The native sequence is regenerated on cleavage and deprotection.
Applications
In recent years, several peptides, such as T-20 (also called DP178), EptifibatideEptifibatide
Eptifibatide , is an antiplatelet drug of the glycoprotein IIb/IIIa inhibitor class. Eptifibatide is a cyclic heptapeptide derived from a protein found in the venom of the southeastern pygmy rattlesnake...
, Ziconotide, Pramlintide
Pramlintide
Pramlintide acetate is a relatively new adjunct for diabetes , developed by Amylin Pharmaceuticals.-Pharmacology:...
, Exenatide
Exenatide
Exenatide is a medication approved in April 2005 for the treatment of diabetes mellitus type 2. It belongs to the group of incretin mimetics and is manufactured by Amylin Pharmaceuticals and Eli Lilly and Company....
, and Bivalirudin
Bivalirudin
Bivalirudin is a specific and reversible direct thrombin inhibitor .1Chemically, it is a synthetic congener of the naturally occurring drug hirudin .Bivalirudin is a DTI that overcomes many limitations seen with indirect thrombin inhibitors, such as heparin...
, have been approved by the U.S. Food and Drug Administration
Food and Drug Administration
The Food and Drug Administration is an agency of the United States Department of Health and Human Services, one of the United States federal executive departments...
and are on the market for use in the treatment of various diseases. More importantly, at the end of 2004, more than 600 peptides were either in development or advanced preclinical phases.
Improvements
Solid-phase synthesis
In chemistry, solid-phase synthesis is a method in which molecules are bound on a bead and synthesized step-by-step in a reactant solution; compared with normal synthesis in a liquid state, it is easier to remove excess reactant or byproduct from the product. In this method, building blocks are...
has relied on polystyrene
Polystyrene
Polystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
-based resins for the synthesis of all kinds of peptides. However, due to their high hydrophobicity, these resins have certain limitations, particularly in the synthesis of complex peptides, and in such cases, polyethylene glycol
Polyethylene glycol
Polyethylene glycol is a polyether compound with many applications from industrial manufacturing to medicine. It has also been known as polyethylene oxide or polyoxyethylene , depending on its molecular weight, and under the tradename Carbowax.-Available forms:PEG, PEO, or POE refers to an...
(PEG)-based resins are often found to give superior results. Another powerful strategy for expediting the assembly of complex peptides is to employ pseudoproline dipeptides. These derivatives disrupt the interactions among chains that are usually the cause of poor coupling yields in aggregated sequences. A large arsenal of chemical tools is now available for the synthesis of almost all peptides up to 40 amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues. However, several small-size peptides and many large peptides and/or proteins are still unavailable by classical methods.
HIV
Human immunodeficiency virus is a lentivirus that causes acquired immunodeficiency syndrome , a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive...
-1 strain The 68 amino acid of RANTES(24-91) has a high propensity to aggregate. The method combines the advantages of the PEG-based ChemMatrix resin and pseudoproline dipeptides.
See also
- ProlineProlineProline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
- PeptidomimeticPeptidomimeticA peptidomimetic is a small protein-like chain designed to mimic a peptide. They typically arise either from modification of an existing peptide, or by designing similar systems that mimic peptides, such as peptoids and β-peptides...
s (such as peptoidPeptoidPeptoids, or poly-N-substituted glycines, are a class of peptidomimetics whose side chains are appended to the nitrogen atom of the peptide backbone, rather than to the α-carbons .-Chemical structure and synthesis:...
s and β-peptideBeta-peptideβ-peptides consist of β amino acids, which have their amino group bonded to the β carbon rather than the α carbon as in the 20 standard biological amino acids. The only commonly naturally occurring β amino acid is β-alanine; although it is used as a component of larger bioactive molecules,...
s) are molecules related to peptides, but with different properties. - Peptide synthesisPeptide synthesisIn organic chemistry, peptide synthesis is the production of peptides, which are organic compounds in which multiple amino acids are linked via amide bonds which are also known as peptide bonds...
- TranslationTranslation (genetics)In molecular biology and genetics, translation is the third stage of protein biosynthesis . In translation, messenger RNA produced by transcription is decoded by the ribosome to produce a specific amino acid chain, or polypeptide, that will later fold into an active protein...
- RibosomeRibosomeA ribosome is a component of cells that assembles the twenty specific amino acid molecules to form the particular protein molecule determined by the nucleotide sequence of an RNA molecule....

