
Ramberg-Bäcklund reaction
Encyclopedia
The Ramberg-Bäcklund Reaction is an organic reaction
converting an α-halo sulfone
into an alkene
in presence of a base
with extrusion of sulfur dioxide
. The reaction is named after the two Swedish chemists Ludwig Ramberg
and Birger Bäcklund. The carbanion formed by deprotonation gives an unstable thiirane dioxide that decomposes with elimination of sulfur dioxide
. This elimination step is considered to be a concerted cycloelimination.
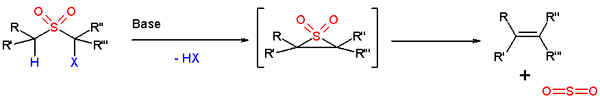 The overall transformation is the conversion of the carbon-sulfur bonds to a carbon-carbon double bond. The original procedure involved halogenation of a sulfide
The overall transformation is the conversion of the carbon-sulfur bonds to a carbon-carbon double bond. The original procedure involved halogenation of a sulfide
, followed by oxidation to the sulfone
. Recently, the preferred method has reversed the order of the steps. After the oxidation, which is normally done with a peroxy acid
, halogenation is done under basic conditions by use of CBr2F2 for the halogen transfer step. This method was used to synthesize 1,8-diphenyl-1,3,5,7-octatraene.
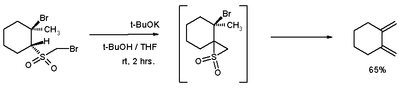
The Ramberg-Bäcklund Reaction has several applications. Due to the nature of elimination, it can be applied to both small rings ,
and large rings containing a double bond .
The necessary α-halo sulfones are accessible through oxidation of the corresponding α-halo sulfides with peracids such as meta-chloroperbenzoic acid; oxidation of sulfides takes place selectively in the presence of alkenes and alcohols. α-Halo sulfides may in turn be synthesized through the treatment of sulfides with halogen electrophiles such as N-chlorosuccinimide.
The sulfone
group contains an acidic proton
in one of the α-positions which is abstracted by a strong base
(scheme 1). The negative charge placed on this position (formally a carbanion
) is transferred to the halogen
residing on the other α-position in a nucleophilic displacement temporarily forming a three-membered cyclic sulfone. This intermediate is unstable and releases sulfur dioxide to form the alkene. Mixtures of cis isomer and trans isomer are usually obtained.
This reaction type gives access to 1,2-dimethylenecyclohexane
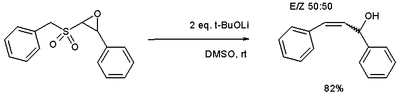
 and the epoxide
and the epoxide
variation access to allyl alcohol
s.
 The Favorskii rearrangement
The Favorskii rearrangement
and the Eschenmoser sulfide contraction
are conceptually related reactions.
A recently developed application of the Ramberg-Bäcklund Reaction is the synthesis of C-glycosides. The required thioethers can be prepared easily by exchange with a thiol
. The application of the Ramberg-Bäcklund conditions then leads to an exocyclic vinyl ether that can be reduced to the C-nucleoside .
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
converting an α-halo sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
into an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
in presence of a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
with extrusion of sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
. The reaction is named after the two Swedish chemists Ludwig Ramberg
Ludwig Ramberg
Ludwig Ramberg was the Swedish chemist who discovered in 1940 the Ramberg-Bäcklund reaction, together with his student Birger Bäcklund .-Life:...
and Birger Bäcklund. The carbanion formed by deprotonation gives an unstable thiirane dioxide that decomposes with elimination of sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
. This elimination step is considered to be a concerted cycloelimination.

Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
, followed by oxidation to the sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
. Recently, the preferred method has reversed the order of the steps. After the oxidation, which is normally done with a peroxy acid
Peroxy acid
A peroxy acid is an acid which contains an acidic -OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the organic derivatives of carboxylic acids...
, halogenation is done under basic conditions by use of CBr2F2 for the halogen transfer step. This method was used to synthesize 1,8-diphenyl-1,3,5,7-octatraene.
The Ramberg-Bäcklund Reaction has several applications. Due to the nature of elimination, it can be applied to both small rings ,
and large rings containing a double bond .
The necessary α-halo sulfones are accessible through oxidation of the corresponding α-halo sulfides with peracids such as meta-chloroperbenzoic acid; oxidation of sulfides takes place selectively in the presence of alkenes and alcohols. α-Halo sulfides may in turn be synthesized through the treatment of sulfides with halogen electrophiles such as N-chlorosuccinimide.
The sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
group contains an acidic proton
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
in one of the α-positions which is abstracted by a strong base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
(scheme 1). The negative charge placed on this position (formally a carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
) is transferred to the halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
residing on the other α-position in a nucleophilic displacement temporarily forming a three-membered cyclic sulfone. This intermediate is unstable and releases sulfur dioxide to form the alkene. Mixtures of cis isomer and trans isomer are usually obtained.
This reaction type gives access to 1,2-dimethylenecyclohexane

Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
variation access to allyl alcohol
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
s.

Favorskii rearrangement
The Favorskii rearrangement , named for the Russian chemist Alexei Yevgrafovich Favorskii, is most principally a rearrangement of cyclopropanones and α-halo ketones which leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorski rearrangement constitutes a ring...
and the Eschenmoser sulfide contraction
Eschenmoser sulfide contraction
The Eschenmoser sulfide contraction is an organic reaction first described by Albert Eschenmoser for the synthesis of 1,3-dicarbonyl compounds from a thioester. The method requires a base and a tertiary phosphine...
are conceptually related reactions.
A recently developed application of the Ramberg-Bäcklund Reaction is the synthesis of C-glycosides. The required thioethers can be prepared easily by exchange with a thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
. The application of the Ramberg-Bäcklund conditions then leads to an exocyclic vinyl ether that can be reduced to the C-nucleoside .

