
Reactive flash volatilization
Encyclopedia
Reactive flash volatilization (RFV) is a chemical process that rapidly converts nonvolatile solids and liquids to volatile compounds by thermal decomposition for integration with catalytic chemistries.
 The utilization of heavy fossil fuels or biomass rich in carbohydrates, (C6H10O5)n, for fuels or chemicals requires an initial thermochemical process called pyrolysis
The utilization of heavy fossil fuels or biomass rich in carbohydrates, (C6H10O5)n, for fuels or chemicals requires an initial thermochemical process called pyrolysis
which fractures large polymers to mixtures of small volatile orgranic compounds (VOCs). A specific method of pyrolysis of biomass, termed "fast pyrolysis," converts particles of biomass to about 10% carbon-rich solid called char
, about 15% gases such as carbon dioxide, and about 70% a mixture of organic compounds commonly referred to as "bio-oil
" at 500 °C in 1–2 seconds. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TFJ-4728F81-1&_user=10&_coverDate=03%2F15%2F2003&_alid=813007377&_rdoc=1&_fmt=high&_orig=search&_cdi=5228&_sort=d&_docanchor=&view=c&_ct=1&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=d9d9d30e965deff86b8334e97156a0b0
Pyrolysis: Biomass + Heat → 0.70VOCs + 0.10Char + 0.15Gases
The volatile organics can be collected as a brown, highly acidic liquid for further thermochemical conversion by traditional processes such as steam reforming
, gasification
, catalytic partial oxidation, catalytic cracking, combustion
, or hydrotreating. http://pubs3.acs.org/acs/journals/doilookup?in_doi=10.1021/cr068360d
Catalytic steam reforming: VOCs + H2O + Heat + Catalyst → H2 + CO + Catalyst
Catalytic partial oxidation: VOCs + O2 + Catalyst → H2 + CO + Heat + Catalyst
Catalytic combustion: VOCs + O2 + Catalyst → CO2 + H2O + Heat + Catalyst
These two sets of chemistries, pyrolysis and catalytic processing, are combined to form the reactive flash volatilization process. Solid hydrocarbons or biomass are contacted with high temperature (500-900 °C) catalysts to generate gases and volatile organic compounds. The volatile species flow into the catalyst with a reactant (H2, O2, or H2O) to convert to desirable products (H2, CO, H2O, CO2, or VOCs).
RFV: Biomass + heat + Reactant + Catalyst → Gases + VOCs + Reactant + Catalyst → Products + Catalyst
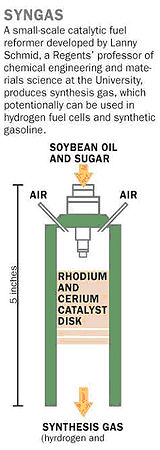 Reactive flash volatilization was demonstrated in 2006 in the journal Science
Reactive flash volatilization was demonstrated in 2006 in the journal Science
by the high temperature (700-800 °C) conversion of soybean oil
(triglycerides) and sugar (D-(+)-glucose) to synthesis gas (H2 + CO) and olefins (ethylene and propylene). Complete, continuous catalytic conversion of heavy fuels was surprising, because the initial pyrolytic chemistry has been shown to generate significant amounts of solid residue called "char" which was expected to block the necessary interaction between the reactant compounds and the solid metal catalyst. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TFJ-4728F81-1&_user=10&_coverDate=03%2F15%2F2003&_alid=812937801&_rdoc=1&_fmt=high&_orig=search&_cdi=5228&_sort=d&_docanchor=&view=c&_ct=1&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=8918a4d862d6ee673bb89901bf9b79f0
The process has been described, "The low volatility of these biofuel feedstocks not only leads to soot production when they are used directly in internal combustion engines but also causes them to coat industrial catalysts with a deactivating layer of carbon, thus hindering their conversion to lighter products. James Richard Salge and colleagues show that if heavy fuels such as soybean oil or biodiesel
are sprayed onto hot rhodium-cerium catalysts as fine droplets in the presence of oxygen, the fuels can self-heat and fully react to form hydrogen without carbon formation and catalyst deactivation."
RFV: Triglyceride + O2 + Catalyst → Ethylene + Propylene + CO2 + H2O + Catalyst
The process converted 70% of the atomic hydrogen in soy-oil triglycerides to molecular H2, and 60% of atomic carbon to carbon monoxide on a Rh-based catalyst with Cerium supported on alpha-alumina. Under different operating conditions, the process can produce a significant amount of ethylene and propylene.
The first demonstration of reactive flash volatilization occurred by a series of experimental steps:
An initial supply of heat is necessary to achieve temperatures of 300 °C, after which the reaction initiates, or "lights off," and quickly rises to temperatures of 700-800 °C. Under steady conditions, the reaction generates sufficient heat to maintain the high temperature, extremely fast chemistry. The total time for conversion of heavy, nonvolatile compounds to volatile or gaseous species occurs in milliseconds (or thousandths of a second). http://www1.umn.edu/umnnews/Feature_Stories/Fuel_in_a_flash.html#
 Reactive flash volatilization of solid particles composed of cellulose
Reactive flash volatilization of solid particles composed of cellulose
, starch
, lignin
, Quaking Aspen (Populus tremuloides
) wood chips, and polyethylene
was demonstrated in 2007 in the scientific journal Angewandte Chemie
. Particles of cellulose were completely converted to syngas
(H2 and CO) and combustion products (H2O and CO2) in as little as 30 milliseconds. Catalytic reforming
of all materials occurred without the requirement of an external heat source while operating at 500-900 °C. Under optimal conditions, 50% of all atomic hydrogen and 50% of all atomic carbon can be converted to molecular H2 and carbon monoxide in as little time as 30 milliseconds. Reaction chemistry was demonstrated on both a Rh-Ce/alumina catalyst and a Ni-Ce/alumina catalyst.
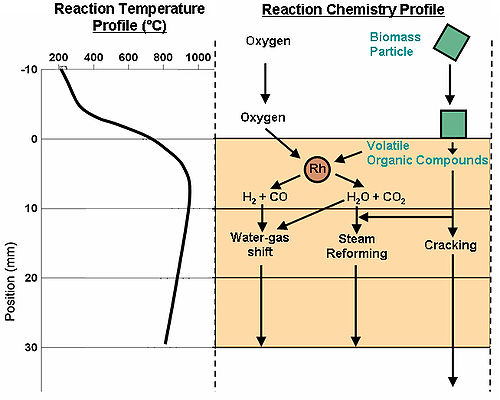
A publication in the scientific journal Green Chemistry
demonstrated that the process of reactive flash volatilization can be considered a combination of several other global chemistries occurring through thermal and chemical integration. As shown in the diagram at the right, the initial pyrolysis chemistry occurs when the biomass particle (green) physically contacts the hot catalyst (orange). Volatile organic compounds (VOCs) flow into the catalyst with oxygen, adsorb on Rh atoms, and react to form combustion products (H2O and CO2) and syngas (H2 and CO). After this initial chemistry, three main global reactions occur. Combustion products react catalytically with syngas by the water-gas shift reaction
. Also, volatile organics react catalytically with steam (H2O) to form new combustion products and syngas. Finally, the volatile organics can crack homogeneously in the gas phase to form smaller volatile organics.
The operating temperature
has been shown to vary within the catalyst length while also being a strong function of the biomass-to-oxygen ratio. An experimental examination has shown that the heat required to thermally fracture biomass was generated within the catalyst bed by surface oxidation reactions. The temperature profile (and reaction temperature) was shown to be extremely important to prevent the formation of carbon at equilibrium. Very fast conversion has been attributed to high operating temperatures, but the maximum cellulose processing rate has not been determined. However, catalytic partial oxidation of volatile organic compounds has shown that complete conversion can occur in less than 10 milliseconds.
Chemistry

Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
which fractures large polymers to mixtures of small volatile orgranic compounds (VOCs). A specific method of pyrolysis of biomass, termed "fast pyrolysis," converts particles of biomass to about 10% carbon-rich solid called char
Char
Char is the solid material that remains after light gases and tar coal tar have been driven out or released from a carbonaceous material during the initial stage of combustion, which is known as carbonization, charring, devolatilization or pyrolysis.Further stages of efficient combustion are...
, about 15% gases such as carbon dioxide, and about 70% a mixture of organic compounds commonly referred to as "bio-oil
Pyrolysis oil
Pyrolysis oil is a synthetic fuel under investigation as substitute for petroleum. It is extracted by biomass to liquid technology of destructive distillation from dried biomass in a reactor at temperature of about 500°C with subsequent cooling. Pyrolytic oil is a kind of tar and normally contains...
" at 500 °C in 1–2 seconds. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TFJ-4728F81-1&_user=10&_coverDate=03%2F15%2F2003&_alid=813007377&_rdoc=1&_fmt=high&_orig=search&_cdi=5228&_sort=d&_docanchor=&view=c&_ct=1&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=d9d9d30e965deff86b8334e97156a0b0
Pyrolysis: Biomass + Heat → 0.70VOCs + 0.10Char + 0.15Gases
The volatile organics can be collected as a brown, highly acidic liquid for further thermochemical conversion by traditional processes such as steam reforming
Steam reforming
Fossil fuel reforming is a method of producing hydrogen or other useful products from fossil fuels such as natural gas. This is achieved in a processing device called a reformer which reacts steam at high temperature with the fossil fuel. The steam methane reformer is widely used in industry to...
, gasification
Gasification
Gasification is a process that converts organic or fossil based carbonaceous materials into carbon monoxide, hydrogen, carbon dioxide and methane. This is achieved by reacting the material at high temperatures , without combustion, with a controlled amount of oxygen and/or steam...
, catalytic partial oxidation, catalytic cracking, combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
, or hydrotreating. http://pubs3.acs.org/acs/journals/doilookup?in_doi=10.1021/cr068360d
Catalytic steam reforming: VOCs + H2O + Heat + Catalyst → H2 + CO + Catalyst
Catalytic partial oxidation: VOCs + O2 + Catalyst → H2 + CO + Heat + Catalyst
Catalytic combustion: VOCs + O2 + Catalyst → CO2 + H2O + Heat + Catalyst
These two sets of chemistries, pyrolysis and catalytic processing, are combined to form the reactive flash volatilization process. Solid hydrocarbons or biomass are contacted with high temperature (500-900 °C) catalysts to generate gases and volatile organic compounds. The volatile species flow into the catalyst with a reactant (H2, O2, or H2O) to convert to desirable products (H2, CO, H2O, CO2, or VOCs).
RFV: Biomass + heat + Reactant + Catalyst → Gases + VOCs + Reactant + Catalyst → Products + Catalyst
Discovery

Science
Science is a systematic enterprise that builds and organizes knowledge in the form of testable explanations and predictions about the universe...
by the high temperature (700-800 °C) conversion of soybean oil
Soybean oil
Soybean oil is a vegetable oil extracted from the seeds of the soybean . It is one of the most widely consumed cooking oils. As a drying oil, processed soybean oil is also used as a base for printing inks and oil paints...
(triglycerides) and sugar (D-(+)-glucose) to synthesis gas (H2 + CO) and olefins (ethylene and propylene). Complete, continuous catalytic conversion of heavy fuels was surprising, because the initial pyrolytic chemistry has been shown to generate significant amounts of solid residue called "char" which was expected to block the necessary interaction between the reactant compounds and the solid metal catalyst. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TFJ-4728F81-1&_user=10&_coverDate=03%2F15%2F2003&_alid=812937801&_rdoc=1&_fmt=high&_orig=search&_cdi=5228&_sort=d&_docanchor=&view=c&_ct=1&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=8918a4d862d6ee673bb89901bf9b79f0
The process has been described, "The low volatility of these biofuel feedstocks not only leads to soot production when they are used directly in internal combustion engines but also causes them to coat industrial catalysts with a deactivating layer of carbon, thus hindering their conversion to lighter products. James Richard Salge and colleagues show that if heavy fuels such as soybean oil or biodiesel
Biodiesel
Biodiesel refers to a vegetable oil- or animal fat-based diesel fuel consisting of long-chain alkyl esters. Biodiesel is typically made by chemically reacting lipids with an alcohol....
are sprayed onto hot rhodium-cerium catalysts as fine droplets in the presence of oxygen, the fuels can self-heat and fully react to form hydrogen without carbon formation and catalyst deactivation."
RFV: Triglyceride + O2 + Catalyst → Ethylene + Propylene + CO2 + H2O + Catalyst
The process converted 70% of the atomic hydrogen in soy-oil triglycerides to molecular H2, and 60% of atomic carbon to carbon monoxide on a Rh-based catalyst with Cerium supported on alpha-alumina. Under different operating conditions, the process can produce a significant amount of ethylene and propylene.
The first demonstration of reactive flash volatilization occurred by a series of experimental steps:
- The researchers start with either pure soybean oil or a thick sugar syrup.
- The reactor consists of an automotive fuel injector, used to spray the oil or syrup as fine droplets through a tube. Sitting like a plug in the tube is a porous ceramic disk made of a rhodium-cerium catalyst material.
- As the droplets hit the disk-whose surface temperature is 1,000 °C-the heat and oxygen break apart the molecules of oil or sugar.
- The catalyst guides the breakdown toward the production of syngas rather than toward water vapor and carbon.
- The syngas passes through the porous disk and is collected downstream in the tube.
- No external heating is needed because the chemical reactions release enough heat to break up molecules of oil or sugar following in their wake.
An initial supply of heat is necessary to achieve temperatures of 300 °C, after which the reaction initiates, or "lights off," and quickly rises to temperatures of 700-800 °C. Under steady conditions, the reaction generates sufficient heat to maintain the high temperature, extremely fast chemistry. The total time for conversion of heavy, nonvolatile compounds to volatile or gaseous species occurs in milliseconds (or thousandths of a second). http://www1.umn.edu/umnnews/Feature_Stories/Fuel_in_a_flash.html#
Application to Solid Biomass

Cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to over ten thousand β linked D-glucose units....
, starch
Starch
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
, lignin
Lignin
Lignin or lignen is a complex chemical compound most commonly derived from wood, and an integral part of the secondary cell walls of plants and some algae. The term was introduced in 1819 by de Candolle and is derived from the Latin word lignum, meaning wood...
, Quaking Aspen (Populus tremuloides
Populus tremuloides
Populus tremuloides is a deciduous tree native to cooler areas of North America, commonly called quaking aspen, trembling aspen, American aspen, and Quakies,. The trees have tall trunks, up to 25 metres, with smooth pale bark, scarred with black. The glossy green leaves, dull beneath, become golden...
) wood chips, and polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
was demonstrated in 2007 in the scientific journal Angewandte Chemie
Angewandte Chemie
Angewandte Chemie is a weekly peer-reviewed scientific journal that covers all aspects of chemistry. Its impact factor was 12.730 in 2010, the highest value for a chemistry-specific journal that publishes original research...
. Particles of cellulose were completely converted to syngas
Syngas
Syngas is the name given to a gas mixture that contains varying amounts of carbon monoxide and hydrogen. Examples of production methods include steam reforming of natural gas or liquid hydrocarbons to produce hydrogen, the gasification of coal, biomass, and in some types of waste-to-energy...
(H2 and CO) and combustion products (H2O and CO2) in as little as 30 milliseconds. Catalytic reforming
Catalytic reforming
Catalytic reforming is a chemical process used to convert petroleum refinery naphthas, typically having low octane ratings, into high-octane liquid products called reformates which are components of high-octane gasoline...
of all materials occurred without the requirement of an external heat source while operating at 500-900 °C. Under optimal conditions, 50% of all atomic hydrogen and 50% of all atomic carbon can be converted to molecular H2 and carbon monoxide in as little time as 30 milliseconds. Reaction chemistry was demonstrated on both a Rh-Ce/alumina catalyst and a Ni-Ce/alumina catalyst.
A publication in the scientific journal Green Chemistry
Green Chemistry
Green Chemistry is a peer-reviewed scientific journal covering any aspect of green chemistry. It is published monthly by the Royal Society of Chemistry and was established in 1999 by James Clark . Articles have to be conceptually accessible to a wide audience...
demonstrated that the process of reactive flash volatilization can be considered a combination of several other global chemistries occurring through thermal and chemical integration. As shown in the diagram at the right, the initial pyrolysis chemistry occurs when the biomass particle (green) physically contacts the hot catalyst (orange). Volatile organic compounds (VOCs) flow into the catalyst with oxygen, adsorb on Rh atoms, and react to form combustion products (H2O and CO2) and syngas (H2 and CO). After this initial chemistry, three main global reactions occur. Combustion products react catalytically with syngas by the water-gas shift reaction
Water gas shift reaction
The water-gas shift reaction is a chemical reaction in which carbon monoxide reacts with water vapor to form carbon dioxide and hydrogen:The water-gas shift reaction is an important industrial reaction. It is often used in conjunction with steam reforming of methane or other hydrocarbons, which is...
. Also, volatile organics react catalytically with steam (H2O) to form new combustion products and syngas. Finally, the volatile organics can crack homogeneously in the gas phase to form smaller volatile organics.
The operating temperature
Operating temperature
An operating temperature is the temperature at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the device function and application context, and ranges from the minimum operating temperature to the...
has been shown to vary within the catalyst length while also being a strong function of the biomass-to-oxygen ratio. An experimental examination has shown that the heat required to thermally fracture biomass was generated within the catalyst bed by surface oxidation reactions. The temperature profile (and reaction temperature) was shown to be extremely important to prevent the formation of carbon at equilibrium. Very fast conversion has been attributed to high operating temperatures, but the maximum cellulose processing rate has not been determined. However, catalytic partial oxidation of volatile organic compounds has shown that complete conversion can occur in less than 10 milliseconds.

