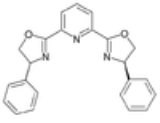
Bisoxazoline ligand
Encyclopedia
In chemistry
, bisoxazoline ligands (BOX ligands for short) are chiral ligand
s based on a bis oxazoline
skeleton and used in combination with a metal
compound in asymmetric synthesis as a chiral catalyst . Three frequently encountered such ligands are PyBOX, tBuBOX and PhBOX. BOX ligands exist as organic compounds with the organometallic complex formed in-situ or as pre-prepared metal complexes.
and William S. Knowles independently developed the first chiral ligands, with those of Knowles based on phosphorus
and those of Noyori based on nitrogen
. One advantage of chiral nitrogen-containing compounds is that they are cheaply available for instance from naturally occurring chiral amino acid
s. The Noyori ligand is based on a reaction of salicylaldehyde
with chiral methylbenzylamine to the imine
followed by complexation
with copper(II) acetate
to the dinuclear copper complex. The cyclopropanation of styrene with ethyl diazoacetate
results in a mixture of cis and trans isomers with a very modest enantiomeric excess
of 6%.
In 1975 Aratani used a similar ligand in the asymmetric cyclopropanation of another diene
to the insecticide
chrysanthemic acid
. His ligand was derived from reaction of salicylaldehyde
with a chiral amino alcohol (the latter synthesized from a Grignard reaction
with an ester of chiral alanine
).
In 1984 Brunner started the development of nitrogen containing ligands because phosphine ligands tended to fail in enantioselective hydrosilylation
s which was the focus of many research groups. One 1984 study describes a nitrogen-nitrogen bidentate ligand with a pyridine
group fused to a thiazolidine
group.
In 1989 Brunner replaced the thiazolidine (difficult to control stereochemistry) by an oxazoline group and demonstrated the new ligand in a monophenylation of a diol
by the organobismuth compound triphenylbismuth diacetate.
In the same year hydrosilylation was demonstrated with a closely related pyridine-oxazoline by Balavoine .
As a logical follow-up step, PyBOX with pyridine flanked by two oxazoline groups, was also introduced in 1989 by Nishiyama . With three nitrogen atoms this ligand is tridentate with bite angle
158.7° in a complex with rhodium(III) chloride
. The isobutyl derivate is synthesized in several steps starting from pyridine-2,6-dicarboxylic acid. The nitrogen fragment is easily obtained from naturally occurring valine
.
BOX-type ligands based on malonate
esters and chiral amino alcohols (obtained by reduction of the corresponding amino acid
s) were first described by in 1990 by Masamune and in 1991 by David Evans.
As before, the ligands are obtained by reaction of an acid chloride with L-valinol (which is the reduced form of valine
) followed by ring-closure to the acetal
and an elimination reaction
(facilitated by either thionyl chloride
or tosyl chloride) to the oxazoline.
Both Masamune and Evans demonstrated their nearly identical BOX ligands in a cyclopropanation reaction via diazotation.
s (not just hydrosilylations or cyclopropanations) are found to display enantioselectivity when performed in presence of a BOX ligand. The obvious targets are 100% enantiomeric excess
(ee) with the use of as little as possible amounts of metal and ligand, usually between 0.01 equivalent and 0.1 equivalent. Selecting the ligand most suitable for a particular reaction is most often a process of trial and error.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, bisoxazoline ligands (BOX ligands for short) are chiral ligand
Chiral ligand
In chemistry a chiral ligand is a specially adapted ligand used for asymmetric synthesis. This ligand is an enantiopure organic compound which combines with a metal center by chelation to form an asymmetric catalyst. This catalyst engages in a chemical reaction and transfers its chirality to the...
s based on a bis oxazoline
Oxazoline
Oxazoline is both the five-membered ring heterocyclic chemical compound with the formula C3H5NO and the class of compounds containing this ring.- See also :* Desoxazoline * Oxazole* Oxazolidine* Oxazolidinedione...
skeleton and used in combination with a metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
compound in asymmetric synthesis as a chiral catalyst . Three frequently encountered such ligands are PyBOX, tBuBOX and PhBOX. BOX ligands exist as organic compounds with the organometallic complex formed in-situ or as pre-prepared metal complexes.
| Bisoxazoline | |R | |Systematic name Systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection... |
CAS number | Melting point Melting point The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure... °C |
|
| PyBOX | phenyl | 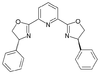 |
2,6-Bis[(4R)-4-phenyl-2-oxazolinyl]pyridine | 128249-70-7 | 171-175 |
| tBuBOX | tert-butyl | 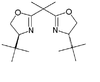 |
(S,S)-2,2′-Methylenebis(4-tert-butyl-2-oxazoline) | 131833-93-7 | 51-53 |
| PhBOX | phenyl | 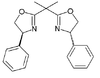 |
(S,S)-2,2-Bis(4-phenyl-2-oxazolin-2-yl)propane | 131457-46-0 | 56-58 |
| Representative bisoxazolines | |||||
Background
In 1968 Ryōji NoyoriRyoji Noyori
is a Japanese chemist. He won the Nobel Prize in Chemistry in 2001. Noyori shared half of the prize with William S. Knowles for the study of chirally catalyzed hydrogenations; the second half of the Prize went to K. Barry Sharpless for his study in chirally catalyzed oxidation reactions...
and William S. Knowles independently developed the first chiral ligands, with those of Knowles based on phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
and those of Noyori based on nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
. One advantage of chiral nitrogen-containing compounds is that they are cheaply available for instance from naturally occurring chiral amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s. The Noyori ligand is based on a reaction of salicylaldehyde
Salicylaldehyde
Salicylaldehyde is the chemical compound with the formula C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration and a characteristic buckwheat...
with chiral methylbenzylamine to the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
followed by complexation
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
with copper(II) acetate
Copper(II) acetate
Copper acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu2 where OAc- is acetate . The hydrated derivative, which contains one molecule of water for each Cu atom, is available commercially. Anhydrous Cu2 is a dark green crystalline solid, whereas Cu22 is...
to the dinuclear copper complex. The cyclopropanation of styrene with ethyl diazoacetate
Ethyl diazoacetate
Ethyl diazoacetate is a diazo compound and a reagent in organic chemistry. It was discovered by Theodor Curtius in 1883. The compound can be prepared by reaction of the ethyl ester of glycine with sodium nitrite and sodium acetate in water....
results in a mixture of cis and trans isomers with a very modest enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
of 6%.
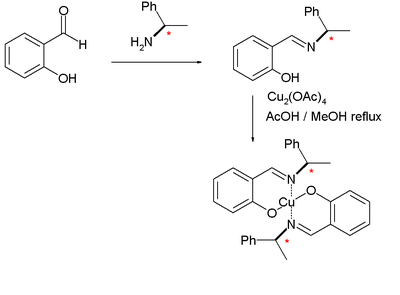 |
|valign=top |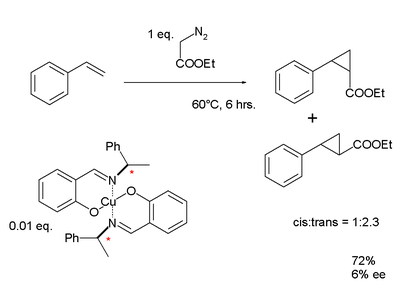 |
|
| Noyori ligand synthesis 1968 | Noyori cyclopropanation 1968 | |
In 1975 Aratani used a similar ligand in the asymmetric cyclopropanation of another diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
to the insecticide
Insecticide
An insecticide is a pesticide used against insects. They include ovicides and larvicides used against the eggs and larvae of insects respectively. Insecticides are used in agriculture, medicine, industry and the household. The use of insecticides is believed to be one of the major factors behind...
chrysanthemic acid
Chrysanthemic acid
Chrysanthemic acid is an organic compound that is related to a variety of natural and synthetic insecticides. It is related to the pyrethrin I and II, as well as the pyrethroids...
. His ligand was derived from reaction of salicylaldehyde
Salicylaldehyde
Salicylaldehyde is the chemical compound with the formula C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration and a characteristic buckwheat...
with a chiral amino alcohol (the latter synthesized from a Grignard reaction
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
with an ester of chiral alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
).
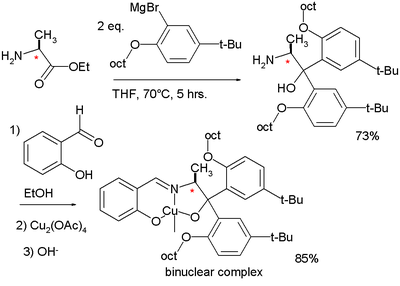 |
|valign=top |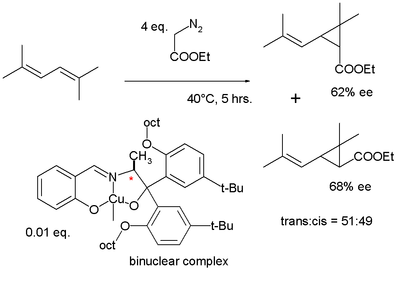 |
|
| Aratani ligand synthesis 1975 | Aratani cyclopropanation 1975 | |
In 1984 Brunner started the development of nitrogen containing ligands because phosphine ligands tended to fail in enantioselective hydrosilylation
Hydrosilylation
Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds. Ordinarily the reaction is conducted catalytically and usually the substrates are unsaturated organic compounds. Alkenes and alkynes give alkyl and vinyl silanes; aldehydes and...
s which was the focus of many research groups. One 1984 study describes a nitrogen-nitrogen bidentate ligand with a pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
group fused to a thiazolidine
Thiazolidine
Thiazolidines are a class of heterocyclic organic compounds with a 5-membered saturated ring with a thioether group and an amine group in the 1 and 3 positions, respectively. It is a sulfur analogue of oxazolidine. The drug pioglitazone contains a thiazolidine ring. It is a drug usually indicated...
group.
In 1989 Brunner replaced the thiazolidine (difficult to control stereochemistry) by an oxazoline group and demonstrated the new ligand in a monophenylation of a diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
by the organobismuth compound triphenylbismuth diacetate.
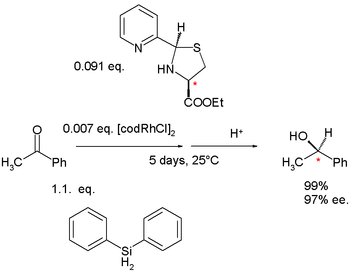 |
|valign=top |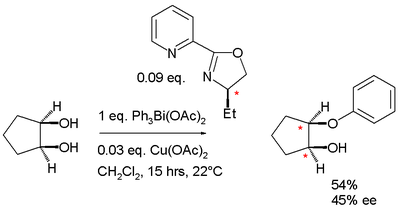 |
|
| Enantioselective hydrosilylation Brunner 1984. | Enantioselective phenylation Brunner 1989 | |
In the same year hydrosilylation was demonstrated with a closely related pyridine-oxazoline by Balavoine .
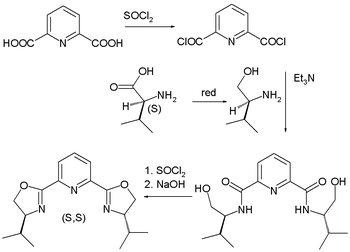
As a logical follow-up step, PyBOX with pyridine flanked by two oxazoline groups, was also introduced in 1989 by Nishiyama . With three nitrogen atoms this ligand is tridentate with bite angle
Bite angle
The bite angle is a geometric parameter used to classify chelating ligands in inorganic and organometallic chemistry. Together with ligand cone angle, this parameter is relevant to diphosphine ligands, which are used in industrial processes such as hydroformylation and hydrocyanation...
158.7° in a complex with rhodium(III) chloride
Rhodium(III) chloride
Rhodium chloride refers to inorganic compounds with the formula RhCl3n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt...
. The isobutyl derivate is synthesized in several steps starting from pyridine-2,6-dicarboxylic acid. The nitrogen fragment is easily obtained from naturally occurring valine
Valine
Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar...
.
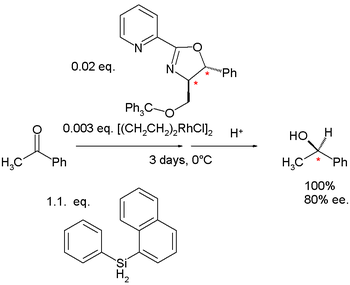 |
|valign=top |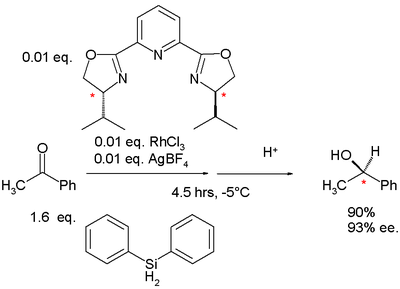 |
|
| Enantioselective hydrosilylation Balavoine 1989 | Enantioselective hydrosilylation Nishiyama 1989 | |
BOX-type ligands based on malonate
Malonate
The malonate or propanedioate ion is CH222− . Malonate compounds include salts and esters of malonic acid, such as*diethyl malonate, 2,*dimethyl malonate, 2,...
esters and chiral amino alcohols (obtained by reduction of the corresponding amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s) were first described by in 1990 by Masamune and in 1991 by David Evans.
 |
|valign=top |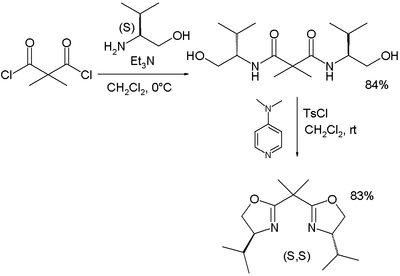 |
|
| PyBOX synthesis Nishiyama 1989 | Bisoxazoline synthesis Evans 1991 | |
As before, the ligands are obtained by reaction of an acid chloride with L-valinol (which is the reduced form of valine
Valine
Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar...
) followed by ring-closure to the acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
and an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
(facilitated by either thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
or tosyl chloride) to the oxazoline.
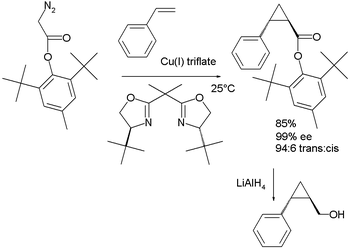
Both Masamune and Evans demonstrated their nearly identical BOX ligands in a cyclopropanation reaction via diazotation.
 |
|valign=top | 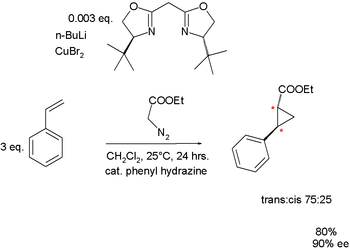 |
|
| Bisoxazoline ligand in cyclopropanation Evans 1991 | Bisoxazoline ligand in cyclopropanation Masamune 1990 | |
Scope
Many organic reactionOrganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s (not just hydrosilylations or cyclopropanations) are found to display enantioselectivity when performed in presence of a BOX ligand. The obvious targets are 100% enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
(ee) with the use of as little as possible amounts of metal and ligand, usually between 0.01 equivalent and 0.1 equivalent. Selecting the ligand most suitable for a particular reaction is most often a process of trial and error.
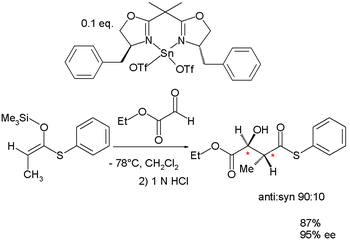 |
|valign=top |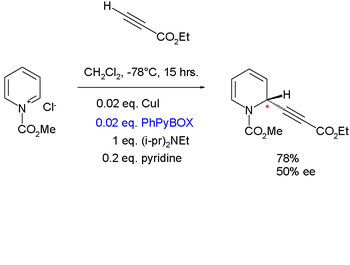 |
|
| Aldol addition | Copper catalyzed nucleophilic addition Nucleophilic addition In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile.... of an alkyne Alkyne Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature... to pyridinium chloride |
|
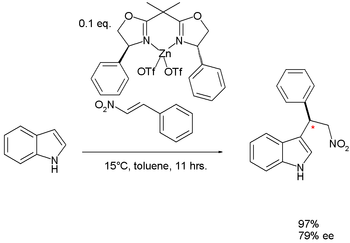 |
|valign=top |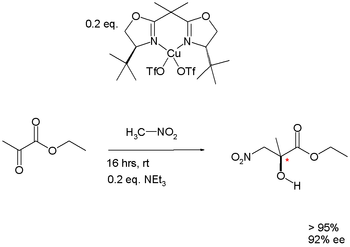 |
|
| Friedel-Crafts alkylation at indole Indole Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an... |
Henry reaction | |
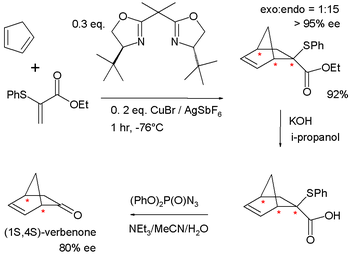 |
|valign=top | 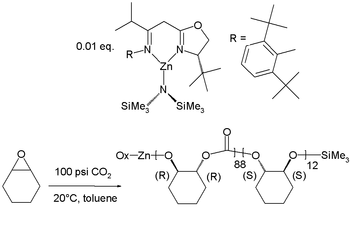 |
|
| Diels-Alder reaction Diels-Alder reaction The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon... resulting in verbenone synthesis |
Copolymerization of cyclohexene oxide and carbon dioxide Carbon dioxide Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom... |
|

