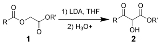
Chan rearrangement
Encyclopedia
The Chan rearrangement is a chemical reaction
that involves rearranging
an acyloxy acetate
(1) in the presence of a strong base
to a 2-hydroxy-3-keto-ester (2).
 This procedure was rediscovered and employed in the Holton Taxol total synthesis
This procedure was rediscovered and employed in the Holton Taxol total synthesis
.
group in the reactant with adjacent carbonyl
and acetyl
substituent
s is acidic and can be deprotonated
by strong non-nucleophilic bases such as lithium tetramethylpiperidide
or lithium diisopropylamide
(LDA) as in an aldol reaction
. The thus formed enolate then gives a nucleophilic acyl substitution
with the adjacent carbonyl
of the acetyl group through a short lived intermediate oxirane. Acidic workup liberates the free hydroxyl group.

Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that involves rearranging
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
an acyloxy acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
(1) in the presence of a strong base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
to a 2-hydroxy-3-keto-ester (2).

Holton Taxol total synthesis
The Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994 was the first total synthesis of Taxol ....
.
Reaction mechanism
The methyleneMethylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
group in the reactant with adjacent carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
and acetyl
Acetyl
In organic chemistry, acetyl is a functional group, the acyl with chemical formula COCH3. It is sometimes represented by the symbol Ac . The acetyl group contains a methyl group single-bonded to a carbonyl...
substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s is acidic and can be deprotonated
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
by strong non-nucleophilic bases such as lithium tetramethylpiperidide
Lithium tetramethylpiperidide
Lithium tetramethylpiperidide or LiTMP is an organic base and a non-nucleophilic base. It is traditionally synthesised from 2,2,6,6-tetramethylpiperidine and n-butyllithium at -78 °C, but recent reports show this reaction is sluggish and is better formed at 0 °C. The compound is stable in a...
or lithium diisopropylamide
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
(LDA) as in an aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
. The thus formed enolate then gives a nucleophilic acyl substitution
Nucleophilic acyl substitution
Nucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...
with the adjacent carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
of the acetyl group through a short lived intermediate oxirane. Acidic workup liberates the free hydroxyl group.


