
Choked flow
Encyclopedia
Choked flow is a compressible flow effect. The parameter that becomes "choked" or "limited" is the velocity or the mass flow rate.
Choked flow is a fluid dynamic
condition associated with the Venturi effect
. When a flowing fluid at a given pressure
and temperature
passes through a restriction (such as the throat of a convergent-divergent nozzle or a valve
in a pipe
) into a lower pressure environment the fluid velocity increases. At initially subsonic upstream conditions, the conservation of mass
principle requires the fluid velocity
to increase as it flows through the smaller cross-sectional area of the restriction. At the same time, the Venturi effect causes the static pressure, and therefore the density, to decrease downstream past the restriction. Choked flow is a limiting condition which occurs when the mass flow rate will not increase with a further decrease in the downstream pressure environment while upstream pressure is fixed.
For homogeneous fluids, the physical point at which the choking occurs for adiabatic
conditions is when the exit plane velocity is at sonic
conditions or at a Mach number
of 1. At choked flow the mass flow rate can be increased by increasing the upstream pressure
, or by decreasing the upstream temperature
.
The choked flow of gases is useful in many engineering applications because the mass flow rate is independent of the downstream pressure, depending only on the temperature and pressure on the upstream side of the restriction. Under choked conditions, valves and calibrated orifice plate
s can be used to produce a desired mass flow rate.
acting on the liquid flow through the restriction decreases the liquid pressure to below that of the liquid vapor pressure
at the prevailing liquid temperature. At that point, the liquid will partially flash
into bubbles of vapor and the subsequent collapse of the bubbles causes cavitation
. Cavitation is quite noisy and can be sufficiently violent to physically damage valves, pipes and associated equipment. In effect, the vapor bubble formation in the restriction limits the flow from increasing any further.
or flow through an orifice plate
.
of the gas (sometimes called the isentropic expansion factor and sometimes denoted as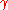 ).
).
For most gases, k ranges from 1.09 (e.g. butane) to 1.67 (monatomic gases), and therefore [ ( k + 1 ) / 2 ] k / ( k - 1 ) ranges from 1.7 to about 2.1 ... which means that choked flow usually occurs when the absolute source vessel pressure is at least 1.7 to 2.1 times as high as the absolute downstream pressure.
When the gas velocity is choked, the equation for the mass flow rate
in SI metric units is:
 =
= 
where the quantities are defined in the table below.
The mass flow rate is primarily dependent on the cross-sectional area A of the hole and the upstream pressure P, and only weakly dependent on the temperature T. The rate does not depend on the downstream pressure at all. All other terms are constants that depend only on the composition of the material in the flow. Although the gas velocity reaches a maximum and becomes choked, the mass flow rate is not choked. The mass flow rate can still be increased if the upstream pressure is increased.
The above equations calculate the steady state mass flow rate for the pressure and temperature existing in the upstream pressure source.
If the gas is being released from a closed high-pressure vessel, the above steady state equations may be used to approximate the initial mass flow rate. Subsequently, the mass flow rate will decrease during the discharge as the source vessel empties and the pressure in the vessel decreases. Calculating the flow rate versus time since the initiation of the discharge is much more complicated, but more accurate. Two equivalent methods for performing such calculations are explained and compared online.
The technical literature can be very confusing because many authors fail to explain whether they are using the universal gas law constant R which applies to any ideal gas
or whether they are using the gas law constant Rs which only applies to a specific individual gas. The relationship between the two constants is Rs = R / M.
Notes:
of the gas. The minimum pressure ratio may be understood as the ratio between the upstream pressure and the pressure at the nozzle throat when the gas is traveling at Mach 1; if the upstream pressure is too low compared to the downstream pressure, sonic flow cannot occur at the throat.
Table 1
Notes:
Inspection of these values leads to the inference that minimum pressure ratio is the following linear function of specific heat ratio: P_ratio = 0.6057 * k + 1.045.
Choked flow is a fluid dynamic
Fluid dynamics
In physics, fluid dynamics is a sub-discipline of fluid mechanics that deals with fluid flow—the natural science of fluids in motion. It has several subdisciplines itself, including aerodynamics and hydrodynamics...
condition associated with the Venturi effect
Venturi effect
The Venturi effect is the reduction in fluid pressure that results when a fluid flows through a constricted section of pipe. The Venturi effect is named after Giovanni Battista Venturi , an Italian physicist.-Background:...
. When a flowing fluid at a given pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
passes through a restriction (such as the throat of a convergent-divergent nozzle or a valve
Valve
A valve is a device that regulates, directs or controls the flow of a fluid by opening, closing, or partially obstructing various passageways. Valves are technically pipe fittings, but are usually discussed as a separate category...
in a pipe
Pipe (material)
A pipe is a tubular section or hollow cylinder, usually but not necessarily of circular cross-section, used mainly to convey substances which can flow — liquids and gases , slurries, powders, masses of small solids...
) into a lower pressure environment the fluid velocity increases. At initially subsonic upstream conditions, the conservation of mass
Conservation of mass
The law of conservation of mass, also known as the principle of mass/matter conservation, states that the mass of an isolated system will remain constant over time...
principle requires the fluid velocity
Velocity
In physics, velocity is speed in a given direction. Speed describes only how fast an object is moving, whereas velocity gives both the speed and direction of the object's motion. To have a constant velocity, an object must have a constant speed and motion in a constant direction. Constant ...
to increase as it flows through the smaller cross-sectional area of the restriction. At the same time, the Venturi effect causes the static pressure, and therefore the density, to decrease downstream past the restriction. Choked flow is a limiting condition which occurs when the mass flow rate will not increase with a further decrease in the downstream pressure environment while upstream pressure is fixed.
For homogeneous fluids, the physical point at which the choking occurs for adiabatic
Adiabatic process
In thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
conditions is when the exit plane velocity is at sonic
Speed of sound
The speed of sound is the distance travelled during a unit of time by a sound wave propagating through an elastic medium. In dry air at , the speed of sound is . This is , or about one kilometer in three seconds or approximately one mile in five seconds....
conditions or at a Mach number
Mach number
Mach number is the speed of an object moving through air, or any other fluid substance, divided by the speed of sound as it is in that substance for its particular physical conditions, including those of temperature and pressure...
of 1. At choked flow the mass flow rate can be increased by increasing the upstream pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, or by decreasing the upstream temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
.
The choked flow of gases is useful in many engineering applications because the mass flow rate is independent of the downstream pressure, depending only on the temperature and pressure on the upstream side of the restriction. Under choked conditions, valves and calibrated orifice plate
Orifice plate
An orifice plate is a device used for measuring the volumetric flow rate. It uses the same principle as a Venturi nozzle, namely Bernoulli's principle which states that there is a relationship between the pressure of the fluid and the velocity of the fluid...
s can be used to produce a desired mass flow rate.
Choked flow in liquids
If the fluid is a liquid, a different type of limiting condition (also known as choked flow) occurs when the Venturi effectVenturi effect
The Venturi effect is the reduction in fluid pressure that results when a fluid flows through a constricted section of pipe. The Venturi effect is named after Giovanni Battista Venturi , an Italian physicist.-Background:...
acting on the liquid flow through the restriction decreases the liquid pressure to below that of the liquid vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
at the prevailing liquid temperature. At that point, the liquid will partially flash
Flash evaporation
Flash evaporation is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations...
into bubbles of vapor and the subsequent collapse of the bubbles causes cavitation
Cavitation
Cavitation is the formation and then immediate implosion of cavities in a liquidi.e. small liquid-free zones that are the consequence of forces acting upon the liquid...
. Cavitation is quite noisy and can be sufficiently violent to physically damage valves, pipes and associated equipment. In effect, the vapor bubble formation in the restriction limits the flow from increasing any further.
Mass flow rate of a gas at choked conditions
All gases flow from upstream higher pressure sources to downstream lower pressure sources. There are several situations in which choked flow occurs, such as the change of cross section in a de Laval nozzleDe Laval nozzle
A de Laval nozzle is a tube that is pinched in the middle, making a carefully balanced, asymmetric hourglass-shape...
or flow through an orifice plate
Orifice plate
An orifice plate is a device used for measuring the volumetric flow rate. It uses the same principle as a Venturi nozzle, namely Bernoulli's principle which states that there is a relationship between the pressure of the fluid and the velocity of the fluid...
.
Choking in change of cross section flow
Assuming ideal gas behavior, steady state choked flow occurs when the ratio of the absolute upstream pressure to the absolute downstream pressure is equal to or greater than [ ( k + 1 ) / 2 ] k / ( k - 1 ), where k is the specific heat ratioHeat capacity ratio
The heat capacity ratio or adiabatic index or ratio of specific heats, is the ratio of the heat capacity at constant pressure to heat capacity at constant volume . It is sometimes also known as the isentropic expansion factor and is denoted by \gamma or \kappa . The latter symbol kappa is...
of the gas (sometimes called the isentropic expansion factor and sometimes denoted as
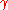 ).
).For most gases, k ranges from 1.09 (e.g. butane) to 1.67 (monatomic gases), and therefore [ ( k + 1 ) / 2 ] k / ( k - 1 ) ranges from 1.7 to about 2.1 ... which means that choked flow usually occurs when the absolute source vessel pressure is at least 1.7 to 2.1 times as high as the absolute downstream pressure.
When the gas velocity is choked, the equation for the mass flow rate
Mass flow rate
Mass flow rate is the mass of substance which passes through a given surface per unit time. Its unit is mass divided by time, so kilogram per second in SI units, and slug per second or pound per second in US customary units...
in SI metric units is:
 =
= 
where the quantities are defined in the table below.
The mass flow rate is primarily dependent on the cross-sectional area A of the hole and the upstream pressure P, and only weakly dependent on the temperature T. The rate does not depend on the downstream pressure at all. All other terms are constants that depend only on the composition of the material in the flow. Although the gas velocity reaches a maximum and becomes choked, the mass flow rate is not choked. The mass flow rate can still be increased if the upstream pressure is increased.
| where: | |
 |
= mass flow rate Mass flow rate Mass flow rate is the mass of substance which passes through a given surface per unit time. Its unit is mass divided by time, so kilogram per second in SI units, and slug per second or pound per second in US customary units... , kg/s |
|---|---|
| C | = discharge coefficient Discharge coefficient In a nozzle or other constriction, the discharge coefficient is the ratio of the mass flow rate at the discharge end of the nozzle to that of an ideal nozzle which expands an identical working fluid from the same initial conditions to the same exit pressures-References:, J. B. Calvert, 15 June 2003... , dimensionless |
| A | = discharge hole cross-sectional area, m² |
| k | = cp/cv of the gas |
| cp | = specific heat of the gas at constant pressure |
| cv | = specific heat of the gas at constant volume |
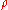 |
= real gas density at P and T, kg/m³ |
| P | = absolute upstream pressure of the gas, Pa |
| M | = the gas molecular mass Molecular mass The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u... , kg/kmole (also known as the molecular weight) |
| R | = Universal gas law constant Gas constant The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,... = 8314.5 (N·m) / (kmole·K) |
| T | = absolute upstream temperature of the gas, K |
| Z | = the gas compressibility factor at P and T, dimensionless |
The above equations calculate the steady state mass flow rate for the pressure and temperature existing in the upstream pressure source.
If the gas is being released from a closed high-pressure vessel, the above steady state equations may be used to approximate the initial mass flow rate. Subsequently, the mass flow rate will decrease during the discharge as the source vessel empties and the pressure in the vessel decreases. Calculating the flow rate versus time since the initiation of the discharge is much more complicated, but more accurate. Two equivalent methods for performing such calculations are explained and compared online.
The technical literature can be very confusing because many authors fail to explain whether they are using the universal gas law constant R which applies to any ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
or whether they are using the gas law constant Rs which only applies to a specific individual gas. The relationship between the two constants is Rs = R / M.
Notes:
- For any ideal gas, Z = 1
- kmole = 1000 molesMole (unit)The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
Real Gas Effects
If the upstream conditions are such that the gas cannot be treated as ideal, there is no closed form equation for evaluating the choked mass flow. Instead, the gas expansion should be calculated by reference to real gas property tables, where the expansion takes place at constant entropy.Thin-plate orifices
The flow of real gases through thin-plate orifices never becomes fully choked. The mass flow rate through the orifice continues to increase as the downstream pressure is lowered to a perfect vacuum, though the mass flow rate increases slowly as the downstream pressure is reduced below the critical pressure. Cunningham (1951) first drew attention to the fact that choked flow will not occur across a standard, thin, square-edged orifice.Minimum pressure ratio required for choked flow to occur
The minimum pressure ratios required for choked conditions to occur (when some typical industrial gases are flowing) are presented in Table 1. The ratios were obtained using the criteria that choked flow occurs when the ratio of the absolute upstream pressure to the absolute downstream pressure is equal to or greater than [ ( k + 1 ) / 2 ] k / ( k - 1 ) , where k is the specific heat ratioHeat capacity ratio
The heat capacity ratio or adiabatic index or ratio of specific heats, is the ratio of the heat capacity at constant pressure to heat capacity at constant volume . It is sometimes also known as the isentropic expansion factor and is denoted by \gamma or \kappa . The latter symbol kappa is...
of the gas. The minimum pressure ratio may be understood as the ratio between the upstream pressure and the pressure at the nozzle throat when the gas is traveling at Mach 1; if the upstream pressure is too low compared to the downstream pressure, sonic flow cannot occur at the throat.
| Gas | k = cp/cv | Minimum Pu/Pd required for choked flow |
|---|---|---|
| Helium | 1.660 | 2.049 |
| Hydrogen | 1.410 | 1.899 |
| Methane | 1.307 | 1.837 |
| Propane | 1.131 | 1.729 |
| Butane | 1.096 | 1.708 |
| Ammonia | 1.310 | 1.838 |
| Chlorine | 1.355 | 1.866 |
| Sulfur dioxide | 1.290 | 1.826 |
| Carbon monoxide | 1.404 | 1.895 |
Notes:
- Pu = absolute upstream gas pressure
- Pd = absolute downstream gas pressure
- k values obtained from:
Inspection of these values leads to the inference that minimum pressure ratio is the following linear function of specific heat ratio: P_ratio = 0.6057 * k + 1.045.
Vacuum Conditions
In the case of upstream air pressure at ambient atmospheric pressure and vacuum conditions down stream of an orifice, both the air velocity and the mass flow rate becomes choked or limited when sonic velocity is reached through the orifice.See also
- Accidental release source termsAccidental release source termsAccidental release source terms are the mathematical equations that quantify the flow rate at which accidental releases of air pollutants into the ambient environment can occur at industrial facilities such as petroleum refineries, petrochemical plants, natural gas processing plants, oil and gas ...
includes mass flow rate equations for non-choked gas flows as well. - Orifice plateOrifice plateAn orifice plate is a device used for measuring the volumetric flow rate. It uses the same principle as a Venturi nozzle, namely Bernoulli's principle which states that there is a relationship between the pressure of the fluid and the velocity of the fluid...
includes derivation of non-choked gas flow equation. - de Laval nozzleDe Laval nozzleA de Laval nozzle is a tube that is pinched in the middle, making a carefully balanced, asymmetric hourglass-shape...
s are Venturi tubes that produce supersonic gas velocities as the tube and the gas are first constricted and then the tube and gas are expanded beyond the choke plane. - Rocket engine nozzlesRocket engine nozzlesA rocket engine nozzle is a propelling nozzle used in a rocket engine to expand and accelerate the combustion gases produced by burning propellants so that the exhaust gases exit the nozzle at hypersonic velocities.-History:...
discusses how to calculate the exit velocity from nozzles used in rocket engines. - Hydraulic jumpHydraulic jumpA hydraulic jump is a phenomenon in the science of hydraulics which is frequently observed in open channel flow such as rivers and spillways. When liquid at high velocity discharges into a zone of lower velocity, a rather abrupt rise occurs in the liquid surface...
.
External links
- Additional accidental release source terms
- Choked flow of gases
- Development of source emission models
- Restriction orifice sizing control Perform orifice plate, restriction orifice sizing calculation for a single phase flow.

