Darzens reaction
Encyclopedia
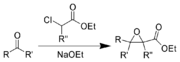
The Darzens reaction is the chemical reaction
of a ketone
or aldehyde
with an α-haloester to form an α,β-epoxy
ester
, also called a "glycidic ester". This reaction was discovered by the organic chemist Auguste George Darzens in 1904.
position. Because of the ester, this carbanion is a resonance
-stabilized enolate, which makes it relatively easy to form. This nucleophilic
structure attacks another carbonyl
component, forming a new carbon–carbon bond. These first two steps are similar to a base-catalyzed aldol reaction
. The oxygen anion in this aldol-like product then does an intramolecular
SN2 attack
on the formerly-nucleophilic halide-bearing position, displacing the halide to form an epoxide. This reaction sequence is thus a condensation reaction
, since there is a net loss of HCl when the two reactant molecules join.
The primary role of the ester is to enable the initial deprotonation to occur, and other carbonyl functional groups can be used instead. If the starting material is an α-halo amide
, the product is an α,β-epoxy amide. If an α-halo ketone is used, the product is an α,β-epoxy ketone.
Any sufficiently strong base can be used for the initial deprotonation. However, if the starting material is an ester, the alkoxide
corresponding to the ester side-chain is commonly in order to prevent complications due to potential acyl
exchange side reactions.
outcome of the reaction is affected by several aspects of the intermediate steps in the sequence.
The initial stereochemistry of the reaction sequence is established in the step where the carbanion attacks the carbonyl. Two sp3
(tetrahedral) carbons are created at this stage, which allows two different diastereomer
ic possibilities of the halohydrin
intermediate. The most likely result is due to chemical kinetics
: whichever product is easier and faster to form will be the major product of this reaction. The subsequent SN2 reaction step proceeds with stereochemical inversion, so the cis or trans form of the epoxide is controlled by the kinetics of an intermediate step. Alternately, the halohydrin can epimerize due to the basic nature of the reaction conditions prior to the SN2 reaction. In this case, the initially formed diastereomer can convert to a different one. This is an equilibrium process
, so the cis or trans form of the epoxide is controlled by chemical thermodynamics
--the product resulting from the more stable diastereomer, regardless of which one was the kinetic result.
of the ester can lead to decarboxylation
, which triggers a rearrangement
of the epoxide into a carbonyl (4). Alternately, other epoxide rearrangements can be induced to form other structures.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
or aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
with an α-haloester to form an α,β-epoxy
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
, also called a "glycidic ester". This reaction was discovered by the organic chemist Auguste George Darzens in 1904.
Reaction mechanism
The reaction process begins when a strong base is used to form a carbanion at the halogenatedHalogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
position. Because of the ester, this carbanion is a resonance
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
-stabilized enolate, which makes it relatively easy to form. This nucleophilic
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
structure attacks another carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
component, forming a new carbon–carbon bond. These first two steps are similar to a base-catalyzed aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
. The oxygen anion in this aldol-like product then does an intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
SN2 attack
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
on the formerly-nucleophilic halide-bearing position, displacing the halide to form an epoxide. This reaction sequence is thus a condensation reaction
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
, since there is a net loss of HCl when the two reactant molecules join.
The primary role of the ester is to enable the initial deprotonation to occur, and other carbonyl functional groups can be used instead. If the starting material is an α-halo amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
, the product is an α,β-epoxy amide. If an α-halo ketone is used, the product is an α,β-epoxy ketone.
Any sufficiently strong base can be used for the initial deprotonation. However, if the starting material is an ester, the alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
corresponding to the ester side-chain is commonly in order to prevent complications due to potential acyl
Acyl
An acyl group is a functional group derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids.In organic chemistry, the acyl group is usually derived from a carboxylic acid . Therefore, it has the formula RCO-, where R represents an alkyl group that is...
exchange side reactions.
Stereochemistry
Depending on the specific structures involved, the epoxide may exist in cis and trans forms. A specific reaction may give only cis, only trans, or a mixture of the two. The specific stereochemicalStereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
outcome of the reaction is affected by several aspects of the intermediate steps in the sequence.
The initial stereochemistry of the reaction sequence is established in the step where the carbanion attacks the carbonyl. Two sp3
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
(tetrahedral) carbons are created at this stage, which allows two different diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
ic possibilities of the halohydrin
Halohydrin
A halohydrin or a haloalcohol is a type of organic compound or functional group in which one carbon atom has a halogen substituent, and an adjacent carbon atom has a hydroxyl substituent. They are derived from alcohols are therefore characterized by the presence of both the hydroxyl functional...
intermediate. The most likely result is due to chemical kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
: whichever product is easier and faster to form will be the major product of this reaction. The subsequent SN2 reaction step proceeds with stereochemical inversion, so the cis or trans form of the epoxide is controlled by the kinetics of an intermediate step. Alternately, the halohydrin can epimerize due to the basic nature of the reaction conditions prior to the SN2 reaction. In this case, the initially formed diastereomer can convert to a different one. This is an equilibrium process
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
, so the cis or trans form of the epoxide is controlled by chemical thermodynamics
Chemical thermodynamics
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics...
--the product resulting from the more stable diastereomer, regardless of which one was the kinetic result.
Alternative reactions
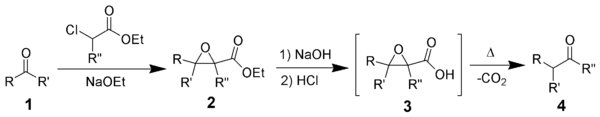
Glycidic esters can also be obtained via nucleophilic epoxidation of an α,β-unsaturated ester, but that approach requires synthesis of the alkene substrate first whereas the Darzens condensation allows formation of the carbon–carbon connectivity and epoxide ring in a single reaction.Subsequent reactions
The product of the Darzens reaction can be reacted further to form various types of compounds. HydrolysisHydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of the ester can lead to decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
, which triggers a rearrangement
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
of the epoxide into a carbonyl (4). Alternately, other epoxide rearrangements can be induced to form other structures.