
Decay chain
Encyclopedia
In nuclear science, the decay chain refers to the radioactive decay
of different discrete radioactive decay product
s as a chained series of transformations. Most radioactive elements do not decay directly to a stable state, but rather undergo a series of decays until eventually a stable isotope
is reached.
Decay stages are referred to by their relationship to previous or subsequent stages. A parent isotope is one that undergoes decay to form a daughter isotope. The daughter isotope may be stable or it may decay to form a daughter isotope of its own. The daughter of a daughter isotope is sometimes called a granddaughter isotope.
The time it takes for a single parent atom to decay to an atom of its daughter isotope can vary widely, not only for different parent-daughter chains, but also for identical pairings of parent and daughter isotopes. While the decay of a single atom occurs spontaneously, the decay of an initial population of identical atoms over time, t, follows a decaying exponential distribution, e−λt, where λ is called a decay constant. Because of this exponential nature, one of the properties of an isotope is its half-life
, the time by which half of an initial number of identical parent radioisotopes have decayed to their daughters. Half-lives have been determined in laboratories for thousands of radioisotopes (or, radionuclide
s). These can range from nearly instantaneous to as much as 1019 years or more.
The intermediate stages often emit more radioactivity than the original radioisotope. When equilibrium is achieved, a granddaughter isotope is present in proportion to its half-life; but since its activity is inversely proportional to its half-life, any nuclide in the decay chain finally contributes as much as the head of the chain. For example, natural uranium
is not significantly radioactive, but pitchblende, a uranium ore, is 13 times more radioactive because of the radium
and other daughter isotopes it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain they also generate radon
, a heavy, inert, naturally occurring radioactive gas. Rock containing thorium and/or uranium (such as some granites) emits radon gas that can accumulate in enclosed places such as basements or underground mines. Radon exposure is considered the leading cause of lung cancer
in non-smokers.
 The four most common modes of radioactive decay are: alpha decay
The four most common modes of radioactive decay are: alpha decay
, beta decay
, inverse beta decay (considered as both positron emission
and electron capture
), and isomeric transition
. Of these decay processes, only alpha decay changes the atomic mass
number (A) of the nucleus, and always decreases it by four. Because of this, almost any decay will result in a nucleus whose atomic mass number has the same residue
mod 4, dividing all nuclides into four classes. The members of any possible decay chain must be drawn entirely from one of these classes. All four chains also produce helium-4
(alpha particles are helium-4 nuclei).
Three main decay chains (or families) are observed in nature, commonly called the thorium
series, the radium
series, and the actinium
series, representing three of these four classes, and ending in three different, stable isotopes of lead
. The mass number of every isotope in these chains can be represented as A = 4n, A = 4n + 2, and A = 4n + 3, respectively. The long-lived starting isotopes of these three isotopes, respectively thorium-232, uranium-238
, and uranium-235
, have existed since the formation of the earth. The plutonium
isotopes plutonium-244
and plutonium-239
have also been found in trace amounts on earth.
Due to the quite short half-life
of its starting isotope neptunium-237 (2.14 million years), the fourth chain, the neptunium
series with A = 4n + 1, is already extinct in nature, except for the final rate-limiting step, decay of bismuth-209
. The ending isotope of this chain is now known to be thallium-205. Some older sources give the final isotope as bismuth-209, but it was recently discovered that it is radioactive, with a half-life of .
There are also many shorter chains, for example that of carbon-14
. On Earth, most of the starting isotopes of these chains are generated by cosmic radiation.
s, alpha particle
s, gamma quanta, neutrino
s, Auger electron
s and X-ray
s) and the recoil nucleus, assuming that the original nucleus was at rest. The letter 'a' represents a year.
In the tables below (except neptunium), the historic names of the naturally occurring nuclides are also given. These names were used at the time when the decay chains were first discovered and investigated. From these historical names one can locate the particular chain to which the nuclide belongs, and replace it with its modern name.
The three naturally-occurring actinide alpha decay chains given below—thorium, uranium/radium (from U-238), and actinium (from U-235)—each ends with its own specific lead isotope (Pb-208, Pb-206, and Pb-207 respectively). All these isotopes are stable and are also present in nature as primordial nuclide
s, but their excess amounts in comparison with lead-204 (which has only a primordial origin) can be used in the technique of uranium-lead dating
to date rocks.
-232, this series includes the following elements: actinium
, bismuth
, lead
, polonium
, radium
, and radon
. All are present, at least transiently, in any natural thorium-containing sample, whether metal, compound, or mineral. The series terminates with lead-208.
and thallium
. A smoke detector
containing an americium
-241 ionization chamber accumulates a significant amount of neptunium
-237 as its americium decays; the following elements are also present in it, at least transiently, as decay products of the neptunium: actinium
, astatine
, bismuth, francium
, lead
, polonium
, protactinium
, radium
, thallium, thorium
, and uranium
. Since this series was only studied more recently, its nuclides do not have historic names.
, this series includes the following elements: astatine
, bismuth
, lead
, polonium
, protactinium
, radium
, radon
, thallium
, and thorium
. All are present, at least transiently, in any natural uranium-containing sample, whether metal, compound, or mineral. The series terminates with lead-206.
.png)
is commonly called the "actinium series". Beginning with the naturally-occurring isotope U-235, this decay series includes the following elements: Actinium
, astatine
, bismuth
, francium
, lead
, polonium
, protactinium
, radium
, radon
, thallium
, and thorium
. All are present, at least transiently, in any sample containing uranium-235, whether metal, compound, ore, or mineral. This series terminates with the stable isotope lead-207.
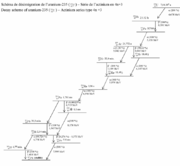
nuclei almost always start out with a neutron/proton ratio significantly greater than what is stable for their mass range. Therefore they undergo multiple beta decay
s in succession, each converting a neutron to a proton. The first decays tend to have higher decay energy and shorter half-life. These last decays may have low decay energy and/or long half-life.
For example, uranium-235
has 92 protons and 143 neutrons. Fission takes one more neutron, then produces two or three more neutrons; assume that 92 protons and 142 neutrons are available for the two fission product nuclei. Suppose they have mass 99 with 39 protons and 60 neutrons (yttrium
-99), and mass 135 with 53 protons and 82 neutrons (iodine
-135), then the decay chains can be found in the tables below.
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
of different discrete radioactive decay product
Decay product
In nuclear physics, a decay product is the remaining nuclide left over from radioactive decay. Radioactive decay often involves a sequence of steps...
s as a chained series of transformations. Most radioactive elements do not decay directly to a stable state, but rather undergo a series of decays until eventually a stable isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
is reached.
Decay stages are referred to by their relationship to previous or subsequent stages. A parent isotope is one that undergoes decay to form a daughter isotope. The daughter isotope may be stable or it may decay to form a daughter isotope of its own. The daughter of a daughter isotope is sometimes called a granddaughter isotope.
The time it takes for a single parent atom to decay to an atom of its daughter isotope can vary widely, not only for different parent-daughter chains, but also for identical pairings of parent and daughter isotopes. While the decay of a single atom occurs spontaneously, the decay of an initial population of identical atoms over time, t, follows a decaying exponential distribution, e−λt, where λ is called a decay constant. Because of this exponential nature, one of the properties of an isotope is its half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
, the time by which half of an initial number of identical parent radioisotopes have decayed to their daughters. Half-lives have been determined in laboratories for thousands of radioisotopes (or, radionuclide
Radionuclide
A radionuclide is an atom with an unstable nucleus, which is a nucleus characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or to an atomic electron. The radionuclide, in this process, undergoes radioactive decay, and emits gamma...
s). These can range from nearly instantaneous to as much as 1019 years or more.
The intermediate stages often emit more radioactivity than the original radioisotope. When equilibrium is achieved, a granddaughter isotope is present in proportion to its half-life; but since its activity is inversely proportional to its half-life, any nuclide in the decay chain finally contributes as much as the head of the chain. For example, natural uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
is not significantly radioactive, but pitchblende, a uranium ore, is 13 times more radioactive because of the radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
and other daughter isotopes it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain they also generate radon
Radon
Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as the decay product of uranium or thorium. Its most stable isotope, 222Rn, has a half-life of 3.8 days...
, a heavy, inert, naturally occurring radioactive gas. Rock containing thorium and/or uranium (such as some granites) emits radon gas that can accumulate in enclosed places such as basements or underground mines. Radon exposure is considered the leading cause of lung cancer
Lung cancer
Lung cancer is a disease characterized by uncontrolled cell growth in tissues of the lung. If left untreated, this growth can spread beyond the lung in a process called metastasis into nearby tissue and, eventually, into other parts of the body. Most cancers that start in lung, known as primary...
in non-smokers.
Types

Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
, beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
, inverse beta decay (considered as both positron emission
Positron emission
Positron emission or beta plus decay is a type of beta decay in which a proton is converted, via the weak force, to a neutron, releasing a positron and a neutrino....
and electron capture
Electron capture
Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
), and isomeric transition
Isomeric transition
An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer....
. Of these decay processes, only alpha decay changes the atomic mass
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
number (A) of the nucleus, and always decreases it by four. Because of this, almost any decay will result in a nucleus whose atomic mass number has the same residue
Modular arithmetic
In mathematics, modular arithmetic is a system of arithmetic for integers, where numbers "wrap around" after they reach a certain value—the modulus....
mod 4, dividing all nuclides into four classes. The members of any possible decay chain must be drawn entirely from one of these classes. All four chains also produce helium-4
Helium-4
Helium-4 is a non-radioactive isotope of helium. It is by far the most abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on earth. Its nucleus is the same as an alpha particle, consisting of two protons and two neutrons. Alpha decay of heavy...
(alpha particles are helium-4 nuclei).
Three main decay chains (or families) are observed in nature, commonly called the thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
series, the radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
series, and the actinium
Actinium
Actinium is a radioactive chemical element with the symbol Ac and atomic number 89, which was discovered in 1899. It was the first non-primordial radioactive element to be isolated. Polonium, radium and radon were observed before actinium, but they were not isolated until 1902...
series, representing three of these four classes, and ending in three different, stable isotopes of lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
. The mass number of every isotope in these chains can be represented as A = 4n, A = 4n + 2, and A = 4n + 3, respectively. The long-lived starting isotopes of these three isotopes, respectively thorium-232, uranium-238
Uranium-238
Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239...
, and uranium-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
, have existed since the formation of the earth. The plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
isotopes plutonium-244
Plutonium-244
Plutonium-244 is an isotope of plutonium that has a halflife of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any actinide except for the three naturally abundant ones uranium-235 , uranium-238, and thorium-232...
and plutonium-239
Plutonium-239
Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in...
have also been found in trace amounts on earth.
Due to the quite short half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of its starting isotope neptunium-237 (2.14 million years), the fourth chain, the neptunium
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
series with A = 4n + 1, is already extinct in nature, except for the final rate-limiting step, decay of bismuth-209
Bismuth-209
Bismuth-209 is the isotope of bismuth with the longest half-life. It has 83 protons and 126 neutrons, and an atomic mass of 208.9803987 u. All primordial bismuth is of this isotope...
. The ending isotope of this chain is now known to be thallium-205. Some older sources give the final isotope as bismuth-209, but it was recently discovered that it is radioactive, with a half-life of .
There are also many shorter chains, for example that of carbon-14
Carbon-14
Carbon-14, 14C, or radiocarbon, is a radioactive isotope of carbon with a nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues , to date archaeological, geological, and hydrogeological...
. On Earth, most of the starting isotopes of these chains are generated by cosmic radiation.
Actinide alpha decay chains
In the four tables below, the minor branches of decay (with the branching ratio of less than 0.0001%) are omitted. The energy release includes the total kinetic energy of all the emitted particles (electronElectron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s, alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
s, gamma quanta, neutrino
Neutrino
A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected...
s, Auger electron
Auger electron
The Auger effect is a physical phenomenon in which the transition of an electron in an atom filling in an inner-shell vacancy causes the emission of another electron. When a core electron is removed, leaving a vacancy, an electron from a higher energy level may fall into the vacancy, resulting in...
s and X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
s) and the recoil nucleus, assuming that the original nucleus was at rest. The letter 'a' represents a year.
In the tables below (except neptunium), the historic names of the naturally occurring nuclides are also given. These names were used at the time when the decay chains were first discovered and investigated. From these historical names one can locate the particular chain to which the nuclide belongs, and replace it with its modern name.
The three naturally-occurring actinide alpha decay chains given below—thorium, uranium/radium (from U-238), and actinium (from U-235)—each ends with its own specific lead isotope (Pb-208, Pb-206, and Pb-207 respectively). All these isotopes are stable and are also present in nature as primordial nuclide
Primordial nuclide
In geochemistry and geonuclear physics, primordial nuclides or primordial isotopes are nuclides found on the earth that have existed in their current form since before Earth was formed. Only 288 such nuclides are known...
s, but their excess amounts in comparison with lead-204 (which has only a primordial origin) can be used in the technique of uranium-lead dating
Uranium-lead dating
Uranium-lead is one of the oldest and most refined of the radiometric dating schemes, with a routine age range of about 1 million years to over 4.5 billion years, and with routine precisions in the 0.1-1 percent range...
to date rocks.
Thorium series
The 4n chain of Th-232 is commonly called the "thorium series." Beginning with naturally occurring thoriumThorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
-232, this series includes the following elements: actinium
Actinium
Actinium is a radioactive chemical element with the symbol Ac and atomic number 89, which was discovered in 1899. It was the first non-primordial radioactive element to be isolated. Polonium, radium and radon were observed before actinium, but they were not isolated until 1902...
, bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
, lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, polonium
Polonium
Polonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie Skłodowska-Curie and Pierre Curie. A rare and highly radioactive element, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Polonium has been studied for...
, radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
, and radon
Radon
Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as the decay product of uranium or thorium. Its most stable isotope, 222Rn, has a half-life of 3.8 days...
. All are present, at least transiently, in any natural thorium-containing sample, whether metal, compound, or mineral. The series terminates with lead-208.
| nuclide | historic name (short) | historic name (long) | decay mode | half-life (a=year) |
energy released, MeV | product of decay |
|---|---|---|---|---|---|---|
| 252Cf | α Alpha decay Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less... |
2.645 a | 6.1181 | 248Cm | ||
| 248Cm | α | 3.4 a | 5.162 | 244Pu Plutonium-244 Plutonium-244 is an isotope of plutonium that has a halflife of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any actinide except for the three naturally abundant ones uranium-235 , uranium-238, and thorium-232... |
||
| 244Pu | α | 8 a | 4.589 | 240U | ||
| 240U | β− Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... |
14.1 h | .39 | 240Np | ||
| 240Np | β− | 1.032 h | 2.2 | 240Pu Plutonium-240 Plutonium-240 is an isotope of the metal plutonium formed when plutonium-239 captures a neutron. About 62% to 73% of the time when Pu-239 captures a neutron it undergoes fission; the rest of the time it forms Pu-240. The longer a nuclear fuel element remains in a nuclear reactor the greater the... |
||
| 240Pu | α | 6561 a | 5.1683 | 236U Uranium-236 - See also :* Depleted uranium* Uranium market* Nuclear reprocessing* United States Enrichment Corporation* Nuclear fuel cycle* Nuclear power-External links:* *... |
||
| 236U | α | 2.3·107 a | 4.494 | 232Th | ||
| 232Th | Th | Thorium | α | 1.405·1010 a | 4.081 | 228Ra |
| 228Ra | MsTh1 | Mesothorium 1 | β− | 5.75 a | 0.046 | 228Ac |
| 228Ac | MsTh2 | Mesothorium 2 | β− | 6.25 h | 2.124 | 228Th |
| 228Th | RdTh | Radiothorium | α | 1.9116 a | 5.520 | 224Ra |
| 224Ra | ThX | Thorium X | α | 3.6319 d | 5.789 | 220Rn |
| 220Rn | Tn | Thoron, Thorium Emanation |
α | 55.6 s | 6.404 | 216Po |
| 216Po | ThA | Thorium A | α | 0.145 s | 6.906 | 212Pb |
| 212Pb | ThB | Thorium B | β− | 10.64 h | 0.570 | 212Bi |
| 212Bi | ThC | Thorium C | β− 64.06% α 35.94% |
60.55 min | 2.252 6.208 |
212Po 208Tl |
| 212Po | ThC' | Thorium C' | α | 299 ns | 8.955 | 208Pb |
| 208Tl | ThC" | Thorium C" | β− | 3.053 min | 4.999 | 208Pb |
| 208Pb | ThD | Thorium D | stable | . | . |
Neptunium series
The 4n + 1 chain of Np-237 is commonly called the "neptunium series." In this series, only two of the elements are found naturally, bismuthBismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
and thallium
Thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. This soft gray poor metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy...
. A smoke detector
Smoke detector
A smoke detector is a device that detects smoke, typically as an indicator of fire. Commercial, industrial, and mass residential devices issue a signal to a fire alarm system, while household detectors, known as smoke alarms, generally issue a local audible and/or visual alarm from the detector...
containing an americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
-241 ionization chamber accumulates a significant amount of neptunium
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
-237 as its americium decays; the following elements are also present in it, at least transiently, as decay products of the neptunium: actinium
Actinium
Actinium is a radioactive chemical element with the symbol Ac and atomic number 89, which was discovered in 1899. It was the first non-primordial radioactive element to be isolated. Polonium, radium and radon were observed before actinium, but they were not isolated until 1902...
, astatine
Astatine
Astatine is a radioactive chemical element with the symbol At and atomic number 85. It occurs on the Earth only as the result of decay of heavier elements, and decays away rapidly, so much less is known about this element than its upper neighbors in the periodic table...
, bismuth, francium
Francium
Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element...
, lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, polonium
Polonium
Polonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie Skłodowska-Curie and Pierre Curie. A rare and highly radioactive element, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Polonium has been studied for...
, protactinium
Protactinium
Protactinium is a chemical element with the symbol Pa and atomic number 91. It is a dense, silvery-gray metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds where protactinium is usually present in the oxidation state +5, but can also assume...
, radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
, thallium, thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
, and uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
. Since this series was only studied more recently, its nuclides do not have historic names.
| nuclide | decay mode | half-life (a=year) |
energy released, MeV | product of decay | |||||
|---|---|---|---|---|---|---|---|---|---|
| 249Cf | α Alpha decay Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less... |
351 a | 5.813+.388 | 245Cm | |||||
| 245Cm | α | 8500 a | 5.362+.175 | 241Pu Plutonium-241 Plutonium-241 is an isotope of plutonium formed when plutonium-240 captures a neutron. Like Pu-239 but unlike 240Pu, 241Pu is fissile, with a neutron absorption cross section about 1/3 greater than 239Pu, and a similar probability of fissioning on neutron absorption, around 73%. In the non-fission... |
|||||
| 241Pu | β− Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... |
14.4 a | 0.021 | 241Am | |||||
| 241Am | α | 432.7 a | 5.638 | 237Np | |||||
| 237Np | α | 2.14·106 a | 4.959 | 233Pa | |||||
| 233Pa | β− | 27.0 d | 0.571 | 233U Uranium-233 Uranium-233 is a fissile isotope of uranium, bred from Thorium as part of the thorium fuel cycle. It has been used in a few nuclear reactors and has been proposed for much wider use as a nuclear fuel. It has a half-life of 160,000 years.... |
|||||
| 233U | α | 1.592·105 a | 4.909 | 229Th |- |
229Th | α | 7340 a | 5.168 | 225Ra |
| 225Ra | β− | 14.9 d | 0.36 | 225Ac | |||||
| 225Ac | α | 10.0 d | 5.935 | 221Fr | |||||
| 221Fr | α | 4.8 min | 6.3 | 217At | |||||
| 217At | α | 32 ms | 7.0 | 213Bi | |||||
| 213Bi | β− 97.80% α 2.20% |
46.5 min | 1.423 5.87 |
213Po 209Tl |
|||||
| 213Po | α | 3.72 μs | 8.536 | 209Pb Lead Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed... |
|||||
| 209Tl | β− | 2.2 min | 3.99 | 209Pb | |||||
| 209Pb | β− | 3.25 h | 0.644 | 209Bi Bismuth-209 Bismuth-209 is the isotope of bismuth with the longest half-life. It has 83 protons and 126 neutrons, and an atomic mass of 208.9803987 u. All primordial bismuth is of this isotope... |
|||||
| 209Bi | α | 1.9·1019 a | 3.14 | 205Tl | |||||
| 205Tl | . | stable | . | . |
Radium series (also known as uranium series)
The 4n+2 chain of U-238 is commonly called the "radium series" (sometimes "uranium series"). Beginning with naturally occurring uranium-238Uranium-238
Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239...
, this series includes the following elements: astatine
Astatine
Astatine is a radioactive chemical element with the symbol At and atomic number 85. It occurs on the Earth only as the result of decay of heavier elements, and decays away rapidly, so much less is known about this element than its upper neighbors in the periodic table...
, bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
, lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, polonium
Polonium
Polonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie Skłodowska-Curie and Pierre Curie. A rare and highly radioactive element, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Polonium has been studied for...
, protactinium
Protactinium
Protactinium is a chemical element with the symbol Pa and atomic number 91. It is a dense, silvery-gray metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds where protactinium is usually present in the oxidation state +5, but can also assume...
, radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
, radon
Radon
Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as the decay product of uranium or thorium. Its most stable isotope, 222Rn, has a half-life of 3.8 days...
, thallium
Thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. This soft gray poor metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy...
, and thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
. All are present, at least transiently, in any natural uranium-containing sample, whether metal, compound, or mineral. The series terminates with lead-206.
.png)
| nuclide | historic name (short) | historic name (long) | decay mode | half-life (a=year) |
energy released, MeV | product of decay |
|---|---|---|---|---|---|---|
| 238U Uranium-238 Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239... |
UI | Uranium I | α Alpha decay Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less... |
4.468·109 a Julian year (astronomy) In astronomy, a Julian year is a unit of measurement of time defined as exactly 365.25 days of 86 400 SI seconds each, totaling 31 557 600 seconds. The Julian year is the average length of the year in the Julian calendar used in Western societies in previous centuries, and for which the unit is... |
4.270 | 234Th |
| 234Th | UX1 | Uranium X1 | β− Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... |
24.10 d Day A day is a unit of time, commonly defined as an interval equal to 24 hours. It also can mean that portion of the full day during which a location is illuminated by the light of the sun... |
0.273 | 234mPa |
| 234mPa | UX2 | Uranium X2, Brevium |
β− 99.84 % IT Isomeric transition An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer.... 0.16 % |
1.16 min Minute A minute is a unit of measurement of time or of angle. The minute is a unit of time equal to 1/60th of an hour or 60 seconds. In the UTC time scale, a minute on rare occasions has 59 or 61 seconds; see leap second. The minute is not an SI unit; however, it is accepted for use with SI units... |
2.271 0.074 |
234U 234Pa |
| 234Pa | UZ | Uranium Z | β− | 6.70 h Hour The hour is a unit of measurement of time. In modern usage, an hour comprises 60 minutes, or 3,600 seconds... |
2.197 | 234U |
| 234U | UII | Uranium II | α | 245500 a | 4.859 | 230Th |
| 230Th | Io | Ionium | α | 75380 a | 4.770 | 226Ra |
| 226Ra | Ra | Radium | α | 1602 a | 4.871 | 222Rn |
| 222Rn | Rn | Radon, Radium Emanation |
α | 3.8235 d | 5.590 | 218Po |
| 218Po | RaA | Radium A | α 99.98 % β− 0.02 % |
3.10 min | 6.115 0.265 |
214Pb 218At |
| 218At | α 99.90 % β− 0.10 % |
1.5 s Second The second is a unit of measurement of time, and is the International System of Units base unit of time. It may be measured using a clock.... |
6.874 2.883 |
214Bi 218Rn |
||
| 218Rn | α | 35 ms 1 E-3 s A millisecond is a thousandth of a second.10 milliseconds are called a centisecond.... |
7.263 | 214Po | ||
| 214Pb | RaB | Radium B | β− | 26.8 min | 1.024 | 214Bi |
| 214Bi | RaC | Radium C | β− 99.98 % α 0.02 % |
19.9 min | 3.272 5.617 |
214Po 210Tl |
| 214Po | RaC' | Radium C' | α | 0.1643 ms | 7.883 | 210Pb |
| 210Tl | RaC" | Radium C" | β− | 1.30 min | 5.484 | 210Pb |
| 210Pb | RaD | Radium D | β− | 22.3 a | 0.064 | 210Bi |
| 210Bi | RaE | Radium E | β− 99.99987% α 0.00013% |
5.013 d | 1.426 5.982 |
210Po 206Tl |
| 210Po | RaF | Radium F | α | 138.376 d | 5.407 | 206Pb |
| 206Tl | RaE" | Radium E" | β− | 4.199 min | 1.533 | 206Pb |
| 206Pb | RaG | Radium G | - | stable | - | - |
Actinium series
The 4n+3 chain of uranium-235Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
is commonly called the "actinium series". Beginning with the naturally-occurring isotope U-235, this decay series includes the following elements: Actinium
Actinium
Actinium is a radioactive chemical element with the symbol Ac and atomic number 89, which was discovered in 1899. It was the first non-primordial radioactive element to be isolated. Polonium, radium and radon were observed before actinium, but they were not isolated until 1902...
, astatine
Astatine
Astatine is a radioactive chemical element with the symbol At and atomic number 85. It occurs on the Earth only as the result of decay of heavier elements, and decays away rapidly, so much less is known about this element than its upper neighbors in the periodic table...
, bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
, francium
Francium
Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element...
, lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, polonium
Polonium
Polonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie Skłodowska-Curie and Pierre Curie. A rare and highly radioactive element, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Polonium has been studied for...
, protactinium
Protactinium
Protactinium is a chemical element with the symbol Pa and atomic number 91. It is a dense, silvery-gray metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds where protactinium is usually present in the oxidation state +5, but can also assume...
, radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
, radon
Radon
Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as the decay product of uranium or thorium. Its most stable isotope, 222Rn, has a half-life of 3.8 days...
, thallium
Thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. This soft gray poor metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy...
, and thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
. All are present, at least transiently, in any sample containing uranium-235, whether metal, compound, ore, or mineral. This series terminates with the stable isotope lead-207.

| nuclide | historic name (short) | historic name (long) | decay mode | half-life (a=year) |
energy released, MeV | product of decay |
|---|---|---|---|---|---|---|
| 239Pu Plutonium-239 Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in... |
α Alpha decay Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less... |
2.41·104 a | 5.244 | 235U | ||
| 235U | AcU | Actin Uranium | α | 7.04·108 a | 4.678 | 231Th |
| 231Th | UY | Uranium Y | β− Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... |
25.52 h | 0.391 | 231Pa |
| 231Pa | Protoactinium | α | 32760 a | 5.150 | 227Ac | |
| 227Ac | Ac | Actinium | β− 98.62% α 1.38% |
21.772 a | 0.045 5.042 |
227Th 223Fr |
| 227Th | RdAc | Radioactinium | α | 18.68 d | 6.147 | 223Ra |
| 223Fr | AcK | Actinium K | β− 99.994% α 0.006% |
22.00 min | 1.149 5.340 |
223Ra 219At |
| 223Ra | AcX | Actinium X | α | 11.43 d | 5.979 | 219Rn |
| 219At | α 97.00% β− 3.00% |
56 s | 6.275 1.700 |
215Bi 219Rn |
||
| 219Rn | An | Actinon, Actinium Emanation |
α | 3.96 s | 6.946 | 215Po |
| 215Bi | β− | 7.6 min | 2.250 | 215Po | ||
| 215Po | AcA | Actinium A | α 99.99977% β− 0.00023% |
1.781 ms | 7.527 0.715 |
211Pb 215At |
| 215At | α | 0.1 ms | 8.178 | 211Bi | ||
| 211Pb | AcB | Actinium B | β− | 36.1 min | 1.367 | 211Bi |
| 211Bi | AcC | Actinium C | α 99.724% β− 0.276% |
2.14 min | 6.751 0.575 |
207Tl 211Po |
| 211Po | AcC' | Actinium C' | α | 516 ms | 7.595 | 207Pb |
| 207Tl | AcC" | Actinium C" | β− | 4.77 min | 1.418 | 207Pb |
| 207Pb | AcD | Actinium D | . | stable | . | . |
Beta decay chains in uranium & plutonium fission products
Since the heavy original nuclei always have a greater proportion of neutrons, the fission productFission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
nuclei almost always start out with a neutron/proton ratio significantly greater than what is stable for their mass range. Therefore they undergo multiple beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
s in succession, each converting a neutron to a proton. The first decays tend to have higher decay energy and shorter half-life. These last decays may have low decay energy and/or long half-life.
For example, uranium-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
has 92 protons and 143 neutrons. Fission takes one more neutron, then produces two or three more neutrons; assume that 92 protons and 142 neutrons are available for the two fission product nuclei. Suppose they have mass 99 with 39 protons and 60 neutrons (yttrium
Yttrium
Yttrium is a chemical element with symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is...
-99), and mass 135 with 53 protons and 82 neutrons (iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
-135), then the decay chains can be found in the tables below.
| Nuclide | Half-life |
|---|---|
| 90Kr | 32.32(9) s |
| 90mRb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
158(5) s |
| 90Rb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
5.84(2) s |
| 90Sr | 28.90(3) a |
| 90mY Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
3.19(6) h |
| 90Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
64.053(20) h |
| 90m2Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
131(4) ns |
| 90m1Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
809.2(20) ms |
| 90Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
stable |
| Nuclide | Half-life |
|---|---|
| 91Kr | 8.57(4) s |
| 91Rb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
58.4(4) s |
| 91Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
9.63(5) h |
| 91mY Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
49.71(4) min |
| 91Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
58.51(6) d |
| 91mZr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
4.35(14) µs |
| 91Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
stable |
| Nuclide | Half-life |
|---|---|
| 92Kr | 1.840(8) s |
| 92Rb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
4.492(20) s |
| 92Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
2.66(4) h |
| 92Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
3.54(1) h |
| 92Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
stable |
| Nuclide | Half-life |
|---|---|
| 93Kr | 1.286(10) s |
| 93mRb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
57(15) µs |
| 93Rb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
5.84(2) s |
| 93Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
7.423(24) min |
| 93mY Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
820(40) ms |
| 93Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
10.18(8) h |
| 93Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
1.53(10)E+6 a |
| 93mNb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
16.13(14) a |
| 93Nb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
stable |
| Nuclide | Half-life |
|---|---|
| 94Kr Isotopes of krypton There are 33 known isotopes of krypton from 69 to 101. Naturally occurring krypton is made of six stable isotopes, two of which may be slightly radioactive. Its spectral signature can be produced with some very sharp lines. 81Kr, the product of atmospheric reactions is produced with the other... |
210(4) ms |
| 94Rb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
2.702(5) s |
| 94Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
75.3(2) s |
| 94Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
18.7(1) min |
| 94Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
stable >1.1E+17 a |
| 94mNb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
6.263(4) min |
| 94Nb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
2.03(16)E+4 a |
| 94Mo Isotopes of molybdenum There are 33 known isotopes of molybdenum ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. Of these naturally occurring isotopes, six have never been observed to decay, but all... |
stable |
| Nuclide | Half-life |
|---|---|
| 95Kr Isotopes of krypton There are 33 known isotopes of krypton from 69 to 101. Naturally occurring krypton is made of six stable isotopes, two of which may be slightly radioactive. Its spectral signature can be produced with some very sharp lines. 81Kr, the product of atmospheric reactions is produced with the other... |
114(3) ms |
| 95Rb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
377.5(8) ms |
| 95Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
23.90(14) s |
| 95Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
10.3(1) min |
| 95Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
64.032(6) d |
| 95mNb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
3.61(3) d |
| 95Nb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
34.991(6) d |
| 95Mo Isotopes of molybdenum There are 33 known isotopes of molybdenum ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. Of these naturally occurring isotopes, six have never been observed to decay, but all... |
stable |
| Nuclide | Half-life |
|---|---|
| 96Kr Isotopes of krypton There are 33 known isotopes of krypton from 69 to 101. Naturally occurring krypton is made of six stable isotopes, two of which may be slightly radioactive. Its spectral signature can be produced with some very sharp lines. 81Kr, the product of atmospheric reactions is produced with the other... |
80(7) ms |
| 96mRb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
200# ms [>1 ms] |
| 96Rb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
202.8(33) ms |
| 96Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
1.07(1) s |
| 96mY Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
9.6(2) s |
| 96Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
5.34(5) s |
| 96Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
20(4)E+18 a |
| 96Nb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
23.35(5) h |
| 96Mo Isotopes of molybdenum There are 33 known isotopes of molybdenum ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. Of these naturally occurring isotopes, six have never been observed to decay, but all... |
stable |
| Nuclide | Half-life |
|---|---|
| 97Kr Isotopes of krypton There are 33 known isotopes of krypton from 69 to 101. Naturally occurring krypton is made of six stable isotopes, two of which may be slightly radioactive. Its spectral signature can be produced with some very sharp lines. 81Kr, the product of atmospheric reactions is produced with the other... |
63(4) ms |
| 97Rb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
169.9(7) ms |
| 97m2Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
255(10) ns |
| 97m1Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
170(10) ns |
| 97Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
429(5) ms |
| 97m2Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
142(8) ms |
| 97m1Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
1.17(3) s |
| 97Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
3.75(3) s |
| 97Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
16.744(11) h |
| 97mNb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
52.7(18) s |
| 97Nb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
72.1(7) min |
| 97Mo Isotopes of molybdenum There are 33 known isotopes of molybdenum ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. Of these naturally occurring isotopes, six have never been observed to decay, but all... |
stable |
| Nuclide | Half-life |
|---|---|
| 98Kr Isotopes of krypton There are 33 known isotopes of krypton from 69 to 101. Naturally occurring krypton is made of six stable isotopes, two of which may be slightly radioactive. Its spectral signature can be produced with some very sharp lines. 81Kr, the product of atmospheric reactions is produced with the other... |
46(8) ms |
| 98mRb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
96(3) ms |
| 98Rb Isotopes of rubidium Rubidium has 32 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb and the radioactive 87Rb . Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. Standard atomic mass is 85.4678 u.87Rb has a half-life of... |
114(5) ms |
| 98Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
0.653(2) s |
| 98m4Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
0.83(10) µs |
| 98m3Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
7.6(4) µs |
| 98m2Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
2.0(2) s |
| 98m1Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
620(80) ns |
| 98Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
0.548(2) s |
| 98Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
30.7(4) s |
| 98mNb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
51.3(4) min |
| 98Nb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
2.86(6) s |
| 98Mo Isotopes of molybdenum There are 33 known isotopes of molybdenum ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. Of these naturally occurring isotopes, six have never been observed to decay, but all... |
stable [>100E+12 a] |
| Nuclide | Half-life |
|---|---|
| 99Sr Isotopes of strontium The alkaline earth metal strontium has four stable, naturally occurring isotopes:84Sr , 86Sr , 87Sr and 88Sr . It has a standard atomic mass of 87.62 u.... |
0.269(1) s |
| 99mY Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
8.6(8) µs |
| 99Y Isotopes of yttrium Natural yttrium is composed of only one isotope 89Y. The most stable radioisotopes are 88Y which has a half-life of 106.65 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours... |
1.470(7) s |
| 99Zr Isotopes of zirconium Naturally occurring zirconium is composed of four stable isotopes , and one very long-lived radioisotope , a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay which is not yet observed, but the theoretically... |
2.1(1) s |
| 99mNb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
2.6(2) min |
| 99Nb Isotopes of niobium Naturally occurring niobium , element 41, is composed of one stable isotope . 93Nb is the lightest nuclide theoretically susceptible to spontaneous fission, and although this has never been observed, it makes niobium theoretically the lightest element with no stable isotope... |
15.0(2) s |
| 99m2Mo Isotopes of molybdenum There are 33 known isotopes of molybdenum ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. Of these naturally occurring isotopes, six have never been observed to decay, but all... |
0.76(6) µs |
| 99m1Mo Isotopes of molybdenum There are 33 known isotopes of molybdenum ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. Of these naturally occurring isotopes, six have never been observed to decay, but all... |
15.5(2) µs |
| 99Mo Isotopes of molybdenum There are 33 known isotopes of molybdenum ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. Of these naturally occurring isotopes, six have never been observed to decay, but all... |
2.7489(6) d |
| 99mTc | 6.0058(12) h |
| 99Tc | 2.111(12)E+5 a |
| 99Ru Isotopes of ruthenium Naturally occurring ruthenium is composed of seven stable isotopes. Additionally, 27 radioactive isotopes have been discovered. Of these radioisotopes, the most stable are 106Ru with a half-life of 373.59 days, 103Ru with a half-life of 39.26 days and 97Ru with a half-life of 2.9 days.Twenty-four... |
stable |
| Nuclide | Half-life |
|---|---|
| 135Te | |19.0(2) s |
| 135I | 6.57(2) h |
| 135Xe | 9.14(2) h |
| 135Cs | 2.3(3)E+6 a |
| 135Ba Isotopes of barium Naturally occurring barium is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, recently identified as being unstable by geochemical means... |
stable |
| Nuclide | Half-life |
|---|---|
| 137Te Isotopes of tellurium There are 38 known isotopes and 17 nuclear isomers of tellurium with atomic masses that range from 105 to 142. These are listed in the table below.Naturally occurring tellurium on Earth consists of eight isotopes... |
|2.49(5) s |
| 137I Isotopes of iodine There are 37 known isotopes of iodine and only one, 127I, is stable. Iodine is thus a monoisotopic element.Its longest-lived radioactive isotope, 129I, has a half-life of 15.7 million years, which is far too short for it to exist as a primordial nuclide... |
24.13(12) s |
| 137Xe Isotopes of xenon Naturally occurring xenon is made of nine stable isotopes. Xenon has the second highest number of stable isotopes. Only tin, with 10 stable isotopes, has more... |
3.818(13) min |
| 137Cs Caesium-137 Caesium-137 is a radioactive isotope of caesium which is formed as a fission product by nuclear fission.It has a half-life of about 30.17 years, and decays by beta emission to a metastable nuclear isomer of barium-137: barium-137m . Caesium-137 is a radioactive isotope of caesium which is formed... |
30.1671(13) a |
| 137m2Ba Isotopes of barium Naturally occurring barium is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, recently identified as being unstable by geochemical means... |
0.59(10) µs |
| 137m1Ba Isotopes of barium Naturally occurring barium is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, recently identified as being unstable by geochemical means... |
2.552(1) min |
| 137Ba Isotopes of barium Naturally occurring barium is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, recently identified as being unstable by geochemical means... |
stable |
| Nuclide | Half-life |
|---|---|
| 144Ba Isotopes of barium Naturally occurring barium is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, recently identified as being unstable by geochemical means... |
11.5(2) s |
| 144La Isotopes of lanthanum Naturally occurring lanthanum is composed of one stable and one radioactive isotope, with the stable isotope, 139La, being the most abundant . 38 radioisotopes have been characterized with the most stable being 138La with a half-life of 102×109 years, 137La with a half-life of 60,000 years... |
40.8(4) s |
| 144Ce Isotopes of cerium Naturally occurring cerium is composed of 4 stable isotopes: 136Ce, 138Ce, 140Ce, and 142Ce with 140Ce being the most abundant ; 136Ce, 138Ce, and 142Ce are predicted to undergo double beta decay but this process has never been observed... |
284.91(5) d |
| 144Pr Isotopes of praseodymium Naturally occurring praseodymium is composed of one stable isotope, 141Pr. Thirty-eight radioisotopes have been characterized with the most stable being 143Pr with a half-life of 13.57 days and 142Pr with a half-life of 19.12 hours... |
17.28(5) min |
| 144Nd Isotopes of neodymium Naturally occurring neodymium is composed of 5 stable isotopes, 142Nd, 143Nd, 145Nd, 146Nd and 148Nd, with 142Nd being the most abundant , and 2 radioisotopes, 144Nd and 150Nd... |
2.29(16)E+15 a |
| 144Pm Isotopes of promethium Promethium has no stable isotopes, and does not exist in nature, except in trace quantities as a product of spontaneous fission. It is a synthetic element, first produced in 1945. Thirty-eight radioisotopes have been characterized, with the most stable being 145Pm with a half-life of 17.7 years,... |
363(14) d |
| 144Sm Isotopes of samarium Naturally occurring samarium is composed of five stable isotopes, 144Sm, 149Sm, 150Sm, 152Sm and 154Sm, and two extremely long-lived radioisotopes, 147Sm and 148Sm , with 152Sm being the most abundant... |
stable |
See also
- Nuclear science
- Radioactive decayRadioactive decayRadioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
- Decay productDecay productIn nuclear physics, a decay product is the remaining nuclide left over from radioactive decay. Radioactive decay often involves a sequence of steps...
- Radioisotopes (RadionuclideRadionuclideA radionuclide is an atom with an unstable nucleus, which is a nucleus characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or to an atomic electron. The radionuclide, in this process, undergoes radioactive decay, and emits gamma...
)
External links
- Decay chains
- Government website listing isotopes and decay energies
- National Nuclear Data Center Freely available databases that can be used to check or construct decay chains. Fully referenced.
-
 The Live Chart of Nuclides - IAEA with decay chains, in Java or HTML
The Live Chart of Nuclides - IAEA with decay chains, in Java or HTML

