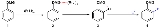
Directed ortho metalation
Encyclopedia
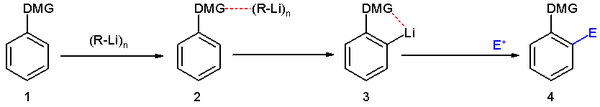
Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution
in which electrophile
s attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium compound . The DMG interacts with lithium through a hetero atom. Examples of DMG's are the methoxy
group, a tertiary amine group and an amide
group.
 The general principle is outlined in scheme 1. An aromatic ring system with a DMG group 1 interacts with an alkyllithium such as n-butyllithium
The general principle is outlined in scheme 1. An aromatic ring system with a DMG group 1 interacts with an alkyllithium such as n-butyllithium
in its specific aggregation state (hence (R-Li)n) to intermediate 2 since the hetero atom on the DMG is a Lewis base and lithium the Lewis acid
. The very basic alkyllithium then deprotonates
the ring in the nearest ortho- position forming the aryllithium 3 all the while maintaining the acid-base interaction. An electrophile reacts in the next phase in an electrophilic aromatic substitution
with a strong preference for the lithium ipso position replacing the lithium atom.
Ordinary electrophilic substitutions with an activating group
show preference for both the ortho and para position, this reaction demonstrates increased regioselectivity
because the ortho position alone is targeted.
 This reaction type was discovered independently by Henry Gilman
This reaction type was discovered independently by Henry Gilman
and Georg Wittig
around 1940.
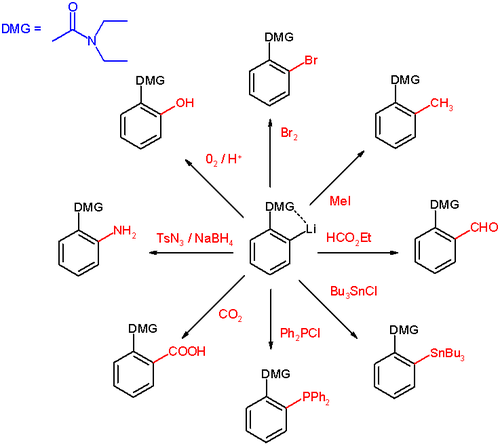
 DOM has been applied to the synthesis of enantiopure benzyl amines in scheme 3 . On approach to the lithium intermediate, the bulky tosyl
DOM has been applied to the synthesis of enantiopure benzyl amines in scheme 3 . On approach to the lithium intermediate, the bulky tosyl
group on the imine
electrophile
is responsible for the asymmetric induction
taking place.
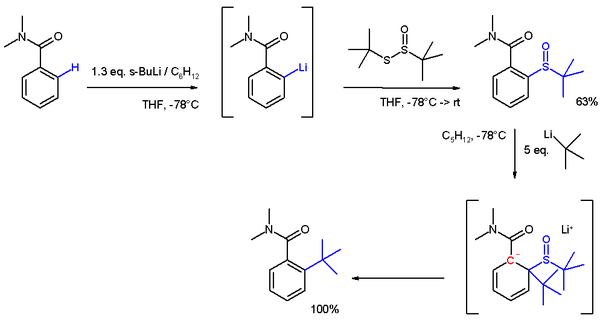
 In another application DOM is applied in placing a bulky tert-butyl group in an ortho position (scheme 4). The lithiation is a nucleophilic aromatic substitution
In another application DOM is applied in placing a bulky tert-butyl group in an ortho position (scheme 4). The lithiation is a nucleophilic aromatic substitution
and the subsequent reaction to the sulfoxide
an electrophilic aromatic substitution
. In the final step tert-butyllithium
acts as a nucleophile
in another nucleophilic aromatic substitution through an anionic intermediate.
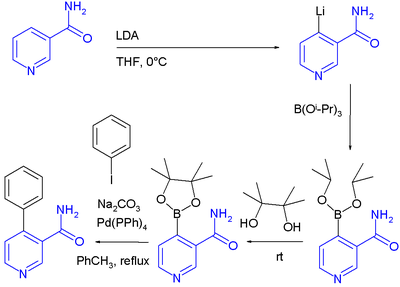
 DoM has also been applied combined with a Suzuki reaction
DoM has also been applied combined with a Suzuki reaction
in a one-pot synthesis
:
, sodium salt of TMP
and di-tert-butylzinc
is a meta zincated complex as a stable crystalline compound. This complex reacts with electrophilic iodine
to N,N-dimethyl-3-iodoaniline :

Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
in which electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium compound . The DMG interacts with lithium through a hetero atom. Examples of DMG's are the methoxy
Methoxy
In chemistry , methoxy refers to the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula O–CH3.The word is used in organic nomenclature usually to describe an ether...
group, a tertiary amine group and an amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
group.

N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
in its specific aggregation state (hence (R-Li)n) to intermediate 2 since the hetero atom on the DMG is a Lewis base and lithium the Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
. The very basic alkyllithium then deprotonates
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
the ring in the nearest ortho- position forming the aryllithium 3 all the while maintaining the acid-base interaction. An electrophile reacts in the next phase in an electrophilic aromatic substitution
Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
with a strong preference for the lithium ipso position replacing the lithium atom.
Ordinary electrophilic substitutions with an activating group
Activating group
In organic chemistry, a functional group is called an activating group if a benzene molecule to which it is attached more readily participates in electrophilic substitution reactions...
show preference for both the ortho and para position, this reaction demonstrates increased regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
because the ortho position alone is targeted.

Henry Gilman
Henry Gilman was an American organic chemist known as the father of organometallic chemistry, the field within which his most notable work was done. He discovered the Gilman reagent, which bears his name....
and Georg Wittig
Georg Wittig
Georg Wittig was a German chemist who reported a method for synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize in Chemistry with Herbert C...
around 1940.
Scope

Tosyl
A tosyl group is CH3C6H4SO2. This group is usually derived from the compound 4-toluenesulfonyl chloride, CH3C6H4SO2Cl, which forms esters and amides of toluenesulfonic or tosylic acid...
group on the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
is responsible for the asymmetric induction
Asymmetric induction
Asymmetric induction in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment...
taking place.

Nucleophilic aromatic substitution
right|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring...
and the subsequent reaction to the sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
an electrophilic aromatic substitution
Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
. In the final step tert-butyllithium
Tert-Butyllithium
tert-Butyllithium is a chemical compound with the formula 3CLi. As an organometallic compound, it has applications in organic synthesis since it is a sufficiently strong base to deprotonate many carbon acids, including benzene...
acts as a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
in another nucleophilic aromatic substitution through an anionic intermediate.

Suzuki reaction
The Suzuki reaction is the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl-halide catalyzed by a palladium complex. It is widely used to synthesize poly-olefins, styrenes, and substituted biphenyls, and has been extended to incorporate alkyl bromides...
in a one-pot synthesis
One-pot synthesis
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor...
:

Related reaction
Directed metallation is not limited to lithium intermediates or even to an ortho preference. In one study it is found that the reaction product of N,N-dimethylaniline with a complex of TMEDATetramethylethylenediamine
Tetramethylethylenediamine is a chemical compound with the formula 2NCH2CH2N2. This species is derived from ethylenediamine by replacement of the four N-H groups with four N-methyl groups. Its odor is remarkably similar to that of fish.-As a reagent in organic and inorganic synthesis:TMEDA is...
, sodium salt of TMP
2,2,6,6-Tetramethylpiperidine
2,2,6,6-Tetramethylpiperidine or TMP or HTMP is a clear liquid with an amine odor. This amine is used in chemistry as a hindered base because it can dissolve in organic solvents unlike ordinary bases such as potassium hydroxide....
and di-tert-butylzinc
Di-tert-butylzinc
Di-tert-butylzinc is a compound with formula ZnC8H18. This compound is used as a meta activating reagent in the syntheses of N,N-dimethyl-3-iodoaniline from N,N-dimethylaniline....
is a meta zincated complex as a stable crystalline compound. This complex reacts with electrophilic iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
to N,N-dimethyl-3-iodoaniline :


