
Equilibrium fractionation
Encyclopedia
Equilibrium isotope fractionation
is the partial separation of isotopes between two or more substances in chemical equilibrium
. Equilibrium fractionation is strongest at low temperatures, and (along with kinetic isotope effects
) forms the basis of the most widely used isotopic paleothermometers
(or climate proxies
): D/H
and 18O/16O
records from ice cores, and 18O/16O records from calcium carbonate. It is thus important for the construction of geologic temperature records
. Isotopic fractionations attributed to equilibrium processes have been observed in many elements, from hydrogen (D/H
) to uranium (238U/235U
). In general, the light elements (especially hydrogen
, boron
, carbon
, nitrogen
, oxygen
and sulfur
) are most susceptible to fractionation, and their isotopes tend to be separated to a greater degree than heavier elements.
Most equilibrium fractionations are thought to result from the reduction in vibrational energy (especially zero-point energy
) when a more massive isotope is substituted for a less massive one. This leads to higher concentrations of the massive isotopes in substances where the vibrational energy is most sensitive to isotope substitution, i.e., those with the highest bond force constants.
In a reaction involving the exchange of two isotopes, lX and hX, of element “X” in molecules
AX and BX,
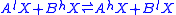
each reactant molecule is identical to a product except for the distribution of isotopes (i.e., they are isotopologues
). The amount of isotopic fractionation in an exchange reaction can be expressed as a fractionation factor:
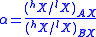
 indicates that the isotopes are distributed evenly between AX and BX, with no isotopic fractionation.
indicates that the isotopes are distributed evenly between AX and BX, with no isotopic fractionation.  indicates that hX is concentrated in substance AX, and
indicates that hX is concentrated in substance AX, and  indicates hX is concentrated in substance BX.
indicates hX is concentrated in substance BX.  is closely related to the equilibrium constant (Keq):
is closely related to the equilibrium constant (Keq):
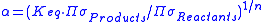
where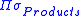 is the product of the rotational symmetry numbers of the products (right side of the exchange reaction),
is the product of the rotational symmetry numbers of the products (right side of the exchange reaction),  is the product of the rotational symmetry numbers of the reactants (left side of the exchange reaction), and
is the product of the rotational symmetry numbers of the reactants (left side of the exchange reaction), and  is the number of atoms exchanged.
is the number of atoms exchanged.
An example of equilibrium isotope fractionation is the concentration of heavy isotopes of oxygen
in liquid water
, relative to water vapor
,
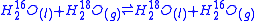
At 20oC, the equilibrium fractionation factor for this reaction is
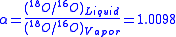
Equilibrium fractionation is a type of mass-dependent isotope fractionation, while mass-independent fractionation
is usually assumed to be a non-equilibrium process.
For non-equilibrium reactions, isotopic effects are better described by the GEBIK and GEBIF equations for transient kinetic isotope fractionation
, which generalize non-steady isotopic effects in any chemical and biochemical reactions.
Isotope geochemistry
Kinetic isotope effect
Isotope analysis
Isotope electrochemistry
δ18O
Kinetic fractionation
Mass-independent fractionation
Transient kinetic isotope effect
Isotopic ratio
Isotopic composition
Fractionation factor
Isotopic enrichment
Fractionation
See also: Fractionated spacecraftFractionation is a separation process in which a certain quantity of a mixture is divided up in a number of smaller quantities in which the composition changes according to a gradient. Fractions are collected based on differences in a specific property of the...
is the partial separation of isotopes between two or more substances in chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
. Equilibrium fractionation is strongest at low temperatures, and (along with kinetic isotope effects
Kinetic isotope effect
The kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
) forms the basis of the most widely used isotopic paleothermometers
Paleothermometer
A paleothermometer is a methodology for determining past temperatures using a proxy found in a natural record such as a sediment, ice core, tree rings or TEX86.=...
(or climate proxies
Proxy (climate)
In the study of past climates is known as paleoclimatology, climate proxies are preserved physical characteristics of the past that stand in for direct measurements , to enable scientists to reconstruct the climatic conditions that prevailed during much of the Earth's history...
): D/H
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
and 18O/16O
Oxygen isotope ratio cycle
Oxygen isotope ratio cycles are cyclical variations in the ratio of the abundance of oxygen with an atomic mass of 18 to the abundance of oxygen with an atomic mass of 16 present in some substances, such as polar ice or calcite in ocean core samples. The ratio is linked to water temperature of...
records from ice cores, and 18O/16O records from calcium carbonate. It is thus important for the construction of geologic temperature records
Geologic temperature record
The Geologic temperature record are changes in Earth's environment as determined from geologic evidence on multi-million to billion year time scales...
. Isotopic fractionations attributed to equilibrium processes have been observed in many elements, from hydrogen (D/H
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
) to uranium (238U/235U
Isotopes of uranium
Uranium is a naturally occurring radioactive element that has no stable isotopes but two primordial isotopes that have long half-life and are found in appreciable quantity in the Earth's crust, along with the decay product uranium-234. The average atomic mass of natural uranium is 238.02891 u...
). In general, the light elements (especially hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
, carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
) are most susceptible to fractionation, and their isotopes tend to be separated to a greater degree than heavier elements.
Most equilibrium fractionations are thought to result from the reduction in vibrational energy (especially zero-point energy
Zero-point energy
Zero-point energy is the lowest possible energy that a quantum mechanical physical system may have; it is the energy of its ground state. All quantum mechanical systems undergo fluctuations even in their ground state and have an associated zero-point energy, a consequence of their wave-like nature...
) when a more massive isotope is substituted for a less massive one. This leads to higher concentrations of the massive isotopes in substances where the vibrational energy is most sensitive to isotope substitution, i.e., those with the highest bond force constants.
In a reaction involving the exchange of two isotopes, lX and hX, of element “X” in molecules
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
AX and BX,
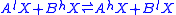
each reactant molecule is identical to a product except for the distribution of isotopes (i.e., they are isotopologues
Isotopologue
Isotopologues are molecules that differ only in their isotopic composition. Simply, the isotopologue of a chemical species has at least one atom with a different number of neutrons than the parent....
). The amount of isotopic fractionation in an exchange reaction can be expressed as a fractionation factor:
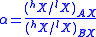
 indicates that the isotopes are distributed evenly between AX and BX, with no isotopic fractionation.
indicates that the isotopes are distributed evenly between AX and BX, with no isotopic fractionation.  indicates that hX is concentrated in substance AX, and
indicates that hX is concentrated in substance AX, and  indicates hX is concentrated in substance BX.
indicates hX is concentrated in substance BX.  is closely related to the equilibrium constant (Keq):
is closely related to the equilibrium constant (Keq):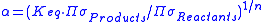
where
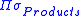 is the product of the rotational symmetry numbers of the products (right side of the exchange reaction),
is the product of the rotational symmetry numbers of the products (right side of the exchange reaction),  is the product of the rotational symmetry numbers of the reactants (left side of the exchange reaction), and
is the product of the rotational symmetry numbers of the reactants (left side of the exchange reaction), and  is the number of atoms exchanged.
is the number of atoms exchanged.An example of equilibrium isotope fractionation is the concentration of heavy isotopes of oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
in liquid water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, relative to water vapor
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
,
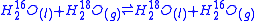
At 20oC, the equilibrium fractionation factor for this reaction is
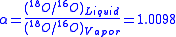
Equilibrium fractionation is a type of mass-dependent isotope fractionation, while mass-independent fractionation
Mass-independent fractionation
Mass-independent fractionation refers to any chemical or physical process that acts to separate isotopes, where the amount of separation does not scale in proportion with the difference in the masses of the isotopes...
is usually assumed to be a non-equilibrium process.
For non-equilibrium reactions, isotopic effects are better described by the GEBIK and GEBIF equations for transient kinetic isotope fractionation
Transient kinetic isotope fractionation
Transient kinetic isotope effects occur when the reaction leading to isotope fractionation does not follow pure first-order kinetics and therefore isotopic effects cannot be described with the classical equilibrium fractionation equations or with steady-state kinetic fractionation equations...
, which generalize non-steady isotopic effects in any chemical and biochemical reactions.
See also
Stable isotopeStable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
Isotope geochemistry
Isotope geochemistry
Isotope geochemistry is an aspect of geology based upon study of the relative and absolute concentrations of the elements and their isotopes in the Earth. Variations in the abundance of these isotopes, typically measured with an isotope ratio mass spectrometer or an accelerator mass spectrometer,...
Kinetic isotope effect
Kinetic isotope effect
The kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
Isotope analysis
Isotope analysis
Isotope analysis is the identification of isotopic signature, the distribution of certain stable isotopes and chemical elements within chemical compounds. This can be applied to a food web to make it possible to draw direct inferences regarding diet, trophic level, and subsistence...
Isotope electrochemistry
Isotope electrochemistry
Isotope electrochemistry is a field within electrochemistry concerned with various topics like electrochemical separation of isotopes, electrochemical estimation of isotopic exchange equilibrium constants , electrochemical kinetic isotope effect, electrochemical isotope sensors, etc.It is an active...
δ18O
Kinetic fractionation
Kinetic fractionation
Kinetic fractionation is a process that separates stable isotopes from each other by their mass during unidirectional processes.One naturally occurring example of kinetic fractionation is the evaporation of seawater to form clouds...
Mass-independent fractionation
Mass-independent fractionation
Mass-independent fractionation refers to any chemical or physical process that acts to separate isotopes, where the amount of separation does not scale in proportion with the difference in the masses of the isotopes...
Transient kinetic isotope effect
Transient kinetic isotope fractionation
Transient kinetic isotope effects occur when the reaction leading to isotope fractionation does not follow pure first-order kinetics and therefore isotopic effects cannot be described with the classical equilibrium fractionation equations or with steady-state kinetic fractionation equations...
Isotopic ratio
Transient kinetic isotope fractionation
Transient kinetic isotope effects occur when the reaction leading to isotope fractionation does not follow pure first-order kinetics and therefore isotopic effects cannot be described with the classical equilibrium fractionation equations or with steady-state kinetic fractionation equations...
Isotopic composition
Transient kinetic isotope fractionation
Transient kinetic isotope effects occur when the reaction leading to isotope fractionation does not follow pure first-order kinetics and therefore isotopic effects cannot be described with the classical equilibrium fractionation equations or with steady-state kinetic fractionation equations...
Fractionation factor
Transient kinetic isotope fractionation
Transient kinetic isotope effects occur when the reaction leading to isotope fractionation does not follow pure first-order kinetics and therefore isotopic effects cannot be described with the classical equilibrium fractionation equations or with steady-state kinetic fractionation equations...
Isotopic enrichment
Transient kinetic isotope fractionation
Transient kinetic isotope effects occur when the reaction leading to isotope fractionation does not follow pure first-order kinetics and therefore isotopic effects cannot be described with the classical equilibrium fractionation equations or with steady-state kinetic fractionation equations...

