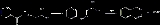
Isoquinoline
Encyclopedia
Isoquinoline is a heterocyclic aromatic organic compound
. It is a structural isomer of quinoline
. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene
ring fused to a pyridine
ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives
. 1-Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine
and morphine
. The isoquinoline ring in these natural compound derives from the aromatic amino acid
tyrosine
.
in water
but dissolve well in ethanol
, acetone
, diethyl ether
, carbon disulfide
, and other common organic solvents. It is also soluble in dilute acid
s as the protonated derivative.
Being an analog
of pyridine
, isoquinoline is a weak base
, with a pKb
of 5.1. It protonates to form salts upon treatment with strong acid
s, such as HCl. It forms adduct
s with Lewis acid
s, such as BF3.
in 1885 by Hoogewerf and van Dorp. They isolated it by fractional crystallization of the acid sulfate. Weissgerber developed a more rapid route in 1914 by selective extraction of coal tar, exploiting the fact that isoquinoline is more basic than quinoline. Isoquinoline can then be isolated from the mixture by fractional crystallization of the acid sulfate.
Although isoquinoline derivatives can be synthesized by several methods, relatively few direct methods deliver the unsubstituted isoquinoline. The Pomeranz-Fritsch reaction provides an efficient method for the preparation of isoquinoline. This reaction uses a benzaldehyde
and aminoacetoaldehyde diethyl acetal, which in an acid
medium
react to form isoquinoline. Alternatively, benzylamine
and a glyoxal
acetal
can be used, to produce the same result.
Several other methods are useful for the preparation of various isoquinoline derivatives.
In the Bischler-Napieralski reaction
an β-phenylethylamine is acylated and cyclodehydrated by a Lewis acid, such as phosphoryl chloride
or phosphorus pentoxide
. The resulting 1-substituted-3,4-dihydroisoquinoline can then be dehydrogenated using palladium. The following Bischler-Napieralski reaction produces papaverine.

The Pictet-Gams synthesis and the Pictet-Spengler reaction
are both variations on the Bischler-Napieralski reaction. A Pictet-Gams reaction works similarly to the Bischler-Napieralski reaction; the only difference being that an elimination of the hydroxy group forms isoquinoline.
In a Pictet-Spengler reaction
, a condensation of a β-phenylethylamine and an aldehyde
forms an imine, which undergoes a cyclization to form a tetrahydroisoquinoline
instead of the dihydroisoquinoline.
Intramolecular aza Wittig reactions also afford isoquinolines.
Isoquinolines find many applications, including (but not limited to):

 Bisbenzylisoquinolinium compounds are compounds similar in structure to tubocurarine
Bisbenzylisoquinolinium compounds are compounds similar in structure to tubocurarine
. They have two isoquinolinium structures, linked by a carbon
chain, containing two ester
linkages.
, a slowly progressing movement disorder, is thought to be caused by certain neurotoxins. A neurotoxin called MPTP
(1[N]-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), the precursor to MPP+, was found and linked to Parkinson's disease in the 1980s. The active neurotoxins destroy dopaminergic neurons, leading to parkinsonism and Parkinson's disease. Several tetrahydroisoquinoline
derivatives have been found to have the same neurochemical properties as MPTP. These derivatives may act as neurotoxin precursors to active neurotoxins.
. It is also used as a solvent
for the extraction
of resins and terpenes, and as a corrosion
inhibitor.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
. It is a structural isomer of quinoline
Quinoline
Quinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
ring fused to a pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives
Derivatization
Derivatization is a technique used in chemistry which transforms a chemical compound into a product of similar chemical structure, called a derivative....
. 1-Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine
Papaverine
Papaverine is an opium alkaloid antispasmodic drug, used primarily in the treatment of visceral spasm, vasospasm , and occasionally in the treatment of erectile dysfunction...
and morphine
Morphine
Morphine is a potent opiate analgesic medication and is considered to be the prototypical opioid. It was first isolated in 1804 by Friedrich Sertürner, first distributed by same in 1817, and first commercially sold by Merck in 1827, which at the time was a single small chemists' shop. It was more...
. The isoquinoline ring in these natural compound derives from the aromatic amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
.
Properties
Isoquinoline is a colorless hygroscopic liquid at room temperature with a penetrating, unpleasant odor. Impure samples can appear brownish, as is typical for nitrogen heterocycles. It crystallizes platelets that have a low solubilitySolubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
in water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
but dissolve well in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
, diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
, carbon disulfide
Carbon disulfide
Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
, and other common organic solvents. It is also soluble in dilute acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s as the protonated derivative.
Being an analog
Analog (chemistry)
In chemistry, a structural analog , also known as chemical analog or simply analog, is a compound having a structure similar to that of another one, but differing from it in respect of a certain component. It can differ in one or more atoms, functional groups, or substructures, which are replaced...
of pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, isoquinoline is a weak base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
, with a pKb
PKB
PKB is a three-letter abbreviation that may refer to:* Państwowy Korpus Bezpieczeństwa, a Polish underground police force during World War II* The National Awakening Party of Indonesia, from the abbreviation for its name in Indonesian...
of 5.1. It protonates to form salts upon treatment with strong acid
Strong acid
A strong acid is an acid that ionizes completely in an aqueous solution by losing one proton, according to the equationFor sulfuric acid which is diprotic, the "strong acid" designation refers only to dissociation of the first protonMore precisely, the acid must be stronger in aqueous solution than...
s, such as HCl. It forms adduct
Adduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
s with Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s, such as BF3.
Production
Isoquinoline was first isolated from coal tarCoal tar
Coal tar is a brown or black liquid of extremely high viscosity, which smells of naphthalene and aromatic hydrocarbons. Coal tar is among the by-products when coal iscarbonized to make coke or gasified to make coal gas...
in 1885 by Hoogewerf and van Dorp. They isolated it by fractional crystallization of the acid sulfate. Weissgerber developed a more rapid route in 1914 by selective extraction of coal tar, exploiting the fact that isoquinoline is more basic than quinoline. Isoquinoline can then be isolated from the mixture by fractional crystallization of the acid sulfate.
Although isoquinoline derivatives can be synthesized by several methods, relatively few direct methods deliver the unsubstituted isoquinoline. The Pomeranz-Fritsch reaction provides an efficient method for the preparation of isoquinoline. This reaction uses a benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
and aminoacetoaldehyde diethyl acetal, which in an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
medium
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
react to form isoquinoline. Alternatively, benzylamine
Benzylamine
Benzylamine is the chemical compound with the formula C6H5CH2NH2. It consists of a benzyl group, C6H5CH2, attached to an amine functional group...
and a glyoxal
Glyoxal
Glyoxal is an organic compound with the formula OCHCHO. This yellow colored liquid is the smallest dialdehyde . Its tautomer acetylenediol is unstable.-Production:...
acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
can be used, to produce the same result.
Several other methods are useful for the preparation of various isoquinoline derivatives.
In the Bischler-Napieralski reaction
Bischler-Napieralski reaction
The Bischler–Napieralski reaction is an intramolecular electrophilic aromatic substitution reaction that allows for the cyclization of β-arylethylamides or β-arylethylcarbamates. It was first discovered in 1893 by August Bischler and Bernard Napieralski, in affiliation with Basle Chemical Works and...
an β-phenylethylamine is acylated and cyclodehydrated by a Lewis acid, such as phosphoryl chloride
Phosphoryl chloride
Phosphoryl chloride is a colourless liquid with the formula 3. It hydrolyses in moist air to phosphoric acid to release choking fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide...
or phosphorus pentoxide
Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
. The resulting 1-substituted-3,4-dihydroisoquinoline can then be dehydrogenated using palladium. The following Bischler-Napieralski reaction produces papaverine.

The Pictet-Gams synthesis and the Pictet-Spengler reaction
Pictet-Spengler reaction
The Pictet–Spengler reaction is a chemical reaction in which a β-arylethylamine such as tryptamine undergoes ringclosure after condensation with an aldehyde or ketone. Usually an acidic catalyst is employed and the reaction mixture heated, but some reactive compounds give good yields even at...
are both variations on the Bischler-Napieralski reaction. A Pictet-Gams reaction works similarly to the Bischler-Napieralski reaction; the only difference being that an elimination of the hydroxy group forms isoquinoline.
In a Pictet-Spengler reaction
Pictet-Spengler reaction
The Pictet–Spengler reaction is a chemical reaction in which a β-arylethylamine such as tryptamine undergoes ringclosure after condensation with an aldehyde or ketone. Usually an acidic catalyst is employed and the reaction mixture heated, but some reactive compounds give good yields even at...
, a condensation of a β-phenylethylamine and an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
forms an imine, which undergoes a cyclization to form a tetrahydroisoquinoline
Tetrahydroisoquinoline
Tetrahydroisoquinoline is a secondary amine with the chemical formula C9H11N.-Reactions:Like other secondary amines, tetrahydroisoquinoline can be oxidized to the corresponding nitrone using hydrogen peroxide, catalyzed by selenium dioxide....
instead of the dihydroisoquinoline.
Intramolecular aza Wittig reactions also afford isoquinolines.
Applications of derivatives
Isoquinolines find many applications, including (but not limited to):
- anesthetics; dimethisoquin is one example (shown below).

- antihypertension agents, such as quinaprilQuinaprilQuinapril is an angiotensin-converting enzyme inhibitor used in the treatment of hypertension and congestive heart failure.-Pharmacology:Quinapril is a prodrug...
, quinapirilat, and debrisoquineDebrisoquineDebrisoquine is a derivative of guanidine. It is an antihypertensive drug similar to guanethidine. Debrisoquine is frequently used for phenotyping the CYP2D6 enzyme, a drug metabolizing enzyme....
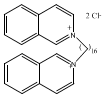
(all derived from 1,2,3,4-tetrahydroisoquinoline). - antifungal agents, such as 2,2'Hexadecamethylenediisoquinolinium dichloride, which is also used as a topical antiseptic. This derivative, shown below, is prepared by N-alkylation of isoquinoline with the appropriate dihalide.

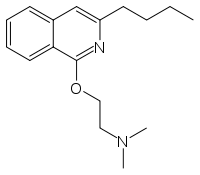
- disinfectants, like N-laurylisoquinolinium bromide (shown below), which is prepared by simple N-alkylation of isoquinoline.

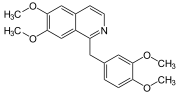
- vasodilators, a well-known example, papaverinePapaverinePapaverine is an opium alkaloid antispasmodic drug, used primarily in the treatment of visceral spasm, vasospasm , and occasionally in the treatment of erectile dysfunction...
, shown below.

Tubocurarine
Tubocurarine is a neuromuscular-blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in anesthesia to provide skeletal muscle relaxation during surgery or mechanical ventilation...
. They have two isoquinolinium structures, linked by a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
chain, containing two ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
linkages.
Isoquinolines and the human body
Parkinson's diseaseParkinson's disease
Parkinson's disease is a degenerative disorder of the central nervous system...
, a slowly progressing movement disorder, is thought to be caused by certain neurotoxins. A neurotoxin called MPTP
MPTP
MPTP is a neurotoxin precursor to MPP+, which causes permanent symptoms of Parkinson's disease by destroying dopaminergic neurons in the substantia nigra of the brain...
(1[N]-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), the precursor to MPP+, was found and linked to Parkinson's disease in the 1980s. The active neurotoxins destroy dopaminergic neurons, leading to parkinsonism and Parkinson's disease. Several tetrahydroisoquinoline
Tetrahydroisoquinoline
Tetrahydroisoquinoline is a secondary amine with the chemical formula C9H11N.-Reactions:Like other secondary amines, tetrahydroisoquinoline can be oxidized to the corresponding nitrone using hydrogen peroxide, catalyzed by selenium dioxide....
derivatives have been found to have the same neurochemical properties as MPTP. These derivatives may act as neurotoxin precursors to active neurotoxins.
Other uses
Isoquinolines are used in the manufacture of dyes, paints, insecticides and antifungalsFungicide
Fungicides are chemical compounds or biological organisms used to kill or inhibit fungi or fungal spores. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality and profit. Fungicides are used both in agriculture and to fight fungal infections in animals...
. It is also used as a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
for the extraction
Liquid-liquid extraction
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid phase into another liquid...
of resins and terpenes, and as a corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
inhibitor.
See also
- QuinolineQuinolineQuinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
, an analog with the nitrogen atom in position 1. - PyridinePyridinePyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, an analog without the fused benzeneBenzeneBenzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
ring. - NaphthaleneNaphthaleneNaphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
, an analog without the nitrogen atom. - Simple aromatic ringSimple aromatic ringSimple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system with delocalized pi electron clouds. Many simple aromatic rings have trivial names. They are usually found as substructures of more complex...
s

