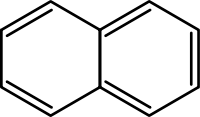
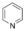
Simple aromatic ring
Encyclopedia
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compound
s that consist only of a conjugated
planar ring system with delocalized
pi electron clouds. Many simple aromatic rings have trivial names. They are usually found as substructures of more complex molecule
s ("substituted aromatics"). Typical simple aromatic compounds are benzene
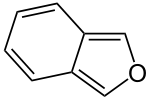
, indole
, and cyclotetradecaheptaene
.
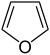
Simple aromatic rings can be heterocyclic if they contain non-carbon
ring atoms, for example, oxygen
, nitrogen
, or sulfur
. They can be monocyclic as in benzene, bicyclic as in naphthalene
, or polycyclic as in anthracene
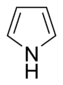
. Simple monocyclic aromatic rings are usually five-membered rings like pyrrole
or six-membered rings like pyridine
. Fused aromatic rings consist of monocyclic rings that share their connecting bonds.
s that are easily protonated
, and form aromatic cations and salts (e.g., pyridinium
), and non-basic aromatic rings.
In the oxygen- and sulfur-containing aromatic rings, one of the electron pairs of the heteroatoms contributes to the aromatic system (similar to the non-basic nitrogen-containing rings), whereas the second lone pair extends in the plane of the ring (similar to the basic nitrogen-containing rings).
In contrast, molecules with 4n pi electrons are antiaromatic.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s that consist only of a conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
planar ring system with delocalized
Delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or one covalent bond....
pi electron clouds. Many simple aromatic rings have trivial names. They are usually found as substructures of more complex molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s ("substituted aromatics"). Typical simple aromatic compounds are benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
, indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
, and cyclotetradecaheptaene
Cyclotetradecaheptaene
Cyclotetradecaheptaene is an aromatic annulene with molecular formula C14H14. It is somewhat unstable due to ring strain....
.
Simple aromatic rings can be heterocyclic if they contain non-carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
ring atoms, for example, oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, or sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
. They can be monocyclic as in benzene, bicyclic as in naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
, or polycyclic as in anthracene
Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon consisting of three fused benzene rings. It is a component of coal-tar. Anthracene is used in the production of the red dye alizarin and other dyes...
. Simple monocyclic aromatic rings are usually five-membered rings like pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
or six-membered rings like pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
. Fused aromatic rings consist of monocyclic rings that share their connecting bonds.
EWLINE
|
|||
Heterocyclic aromatic rings
The nitrogen (N)-containing aromatic rings can be separated into basic aromatic ringBasic aromatic ring
Basic aromatic rings are aromatic rings in which the lone pair of electrons of a ring-nitrogen atom is not part of the aromatic system and extends in the plane of the ring. This lone pair is responsible for the basicity of these nitrogenous bases, similar to the nitrogen atom in amines. In these...
s that are easily protonated
Protonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
, and form aromatic cations and salts (e.g., pyridinium
Pyridinium
Pyridinium refers to the cationic form of pyridine. This can either be due to protonation of the ring nitrogen or because of addition of a substituent to the ring nitrogen, typically via alkylation. The lone pair of electrons on the nitrogen atom of pyridine is not delocalized, and thus pyridine...
), and non-basic aromatic rings.
- In the basic aromatic rings, the lone pairLone pairIn chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
of electronElectronThe electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s is not part of the aromatic system and extends in the plane of the ring. This lone pair is responsible for the basicity of these nitrogenous baseNitrogenous baseA nitrogenous base is a nitrogen-containing molecule having the chemical properties of a base. It is an organic compound that owes its property as a base to the lone pair of electrons of a nitrogen atom. In biological sciences, nitrogenous bases are typically classified as the derivatives of two...
s, similar to the nitrogen atom in amineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s. In these compounds the nitrogen atom is not connected to a hydrogen atom. Examples of basic aromatic rings are pyridinePyridinePyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
or quinolineQuinolineQuinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
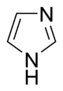
. Several rings contain basic as well as non-basic nitrogen atoms, e.g., imidazoleImidazoleImidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
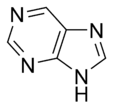
and purinePurineA purine is a heterocyclic aromatic organic compound, consisting of a pyrimidine ring fused to an imidazole ring. Purines, including substituted purines and their tautomers, are the most widely distributed kind of nitrogen-containing heterocycle in nature....
.
- In the non-basic rings, the lone pair of electrons of the nitrogen atom is delocalized and contributes to the aromatic pi electron system. In these compounds, the nitrogen atom is connected to a hydrogen atom. Examples of non-basic nitrogen-containing aromatic rings are pyrrolePyrrolePyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
and indoleIndoleIndole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
.
In the oxygen- and sulfur-containing aromatic rings, one of the electron pairs of the heteroatoms contributes to the aromatic system (similar to the non-basic nitrogen-containing rings), whereas the second lone pair extends in the plane of the ring (similar to the basic nitrogen-containing rings).
Criteria for aromaticity
- Molecule must be cyclic.
- Every atom in the ring must have a p orbital, which overlaps with p orbitals on either side (completely conjugatedConjugated systemIn chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
). - Molecule must be planar.
- It must contain an odd number of pairs of pi electrons; must satisfy Huckel's rule: (4n+2) pi electrons, where n is an integer starting at zero.
In contrast, molecules with 4n pi electrons are antiaromatic.