Lactase
Encyclopedia
Lactase a part of the β-galactosidase family of enzyme
s, is a glycoside hydrolase
involved in the hydrolysis
of the disaccharide
lactose
into constituent galactose
and glucose
monomer
s. Lactase is present predominantly along the brush border
membrane
of the differentiated enterocyte
s lining the villi of the small intestine
. In humans, lactase is encoded by the LCT gene
.
Lactase is essential for digestive hydrolysis of lactose in milk. Deficiency of the enzyme causes lactose intolerance
.
The optimum temperature
for lactase is about 77 °F (25 °C) for its activity and has an optimum pH
of 6.
to phloretin
and glucose.
The catalytic mechanism of D-lactose hydrolysis retains the substrate anomeric configuration in the products. While the details of the mechanism are uncertain, the stereochemical retention is achieved through a double displacement reaction. Studies of E. coli lactase have proposed that hydrolysis is initiated when a glutamate nucleophile on the enzyme attacks from the axial side of the galactosyl carbon in the β-glycosidic bond. The removal of the D-glucose leaving group may be facilitated by Mg-dependent acid catalysis. The enzyme is liberated from the α-galactosyl moiety upon equatorial nucleophilic attack by water, which produces D-galactose.
Substrate modification studies have demonstrated that the 3’-OH and 2’-OH moieties on the galactopyranose ring are essential for enzymatic recognition and hydrolysis. The 3’-hydroxy group is involved in initial binding to the substrate while the 2’- group is not necessary for recognition but needed in subsequent steps. This is demonstrated by the fact that a 2-deoxy analog is an effective competitive inhibitor (Ki = 10mM). Elimination of specific hydroxyl groups on the glucopyranose moiety does not completely eliminate catalysis.
Mature human lactase consists of a single 160 kDa polypeptide chain that localizes to the brush border membrane of intestinal epithelial cells. It is oriented with the N-terminus outside the cell and the C-terminus in the cytosol. LPH contains two catalytic glutamic acid sites. In the human enzyme, the lactase activity has been connected to Glu-1749 while Glu-1273 is the site of phlorizin hydrolase function.
Some population segments exhibit lactase persistence resulting from a mutation that is postulated to have occurred 5000-10,000 years ago, coinciding with the rise of cattle domestication. This mutation has allowed almost half of the world’s population to metabolize lactose without symptoms. Studies have linked the occurrence of lactase persistence to two different single-nucleotide polymorphisms about 14 and 22 kilobases upstream of the 5’-end of the LPH gene. Both mutations, C→T at position -13910 and T→ A at position -22018, have been independently linked to lactase persistence.
The lactase promoter is 150 base pairs long and is located just upstream of the site of transcription initiation. The sequence is highly conserved in mammals, suggesting that critical cis-transcriptional regulators are located nearby. Cdx-2, HNF-1α, and GATA have been identified as transcription factors. Studies of hypolactasia onset have demonstrated that despite polymorphisms there is little difference in lactase expression in infants, showing that the mutations become increasingly relevant during development. Ιt is hypothesized that developmentally-regulated DNA-binding proteins down-regulate transcription or destabilize mRNA transcripts, causing decreased LPH expression after weaning.
s such as Kluyveromyces fragilis and Kluyveromyces lactis
and from fungi
, such as Aspergillus niger
and Aspergillus oryzae
. Its primary commercial use is to break down lactose in milk to make it suitable for people with lactose intolerance
. However, the U.S. Food and Drug Administration (FDA) has not formally evaluated the effectiveness of these products. Lactase is also used in the manufacture of ice cream
. Because glucose and galactose are sweeter than lactose, lactase produces a sweeter ice cream. Lactose also crystallizes at the low temperatures of ice cream; however, its constituent products, glucose
and galactose
, remain dissolved and contribute to a smoother texture. Lactase is used in the conversion of whey
into syrup.
Lactase is also used to screen for blue white
colonies in the MCS
of various plasmid vectors in Escherichia coli
or other bacteria.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s, is a glycoside hydrolase
Glycoside hydrolase
Glycoside hydrolases catalyze the hydrolysis of the glycosidic linkage to release smaller sugars...
involved in the hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of the disaccharide
Disaccharide
A disaccharide or biose is the carbohydrate formed when two monosaccharides undergo a condensation reaction which involves the elimination of a small molecule, such as water, from the functional groups only. Like monosaccharides, disaccharides form an aqueous solution when dissolved in water...
lactose
Lactose
Lactose is a disaccharide sugar that is found most notably in milk and is formed from galactose and glucose. Lactose makes up around 2~8% of milk , although the amount varies among species and individuals. It is extracted from sweet or sour whey. The name comes from or , the Latin word for milk,...
into constituent galactose
Galactose
Galactose , sometimes abbreviated Gal, is a type of sugar that is less sweet than glucose. It is a C-4 epimer of glucose....
and glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s. Lactase is present predominantly along the brush border
Brush border
A brush border is the name for the microvilli-covered surface of simple cuboidal epithelium and simple columnar epithelium cells found in certain locations of the body. Microvilli are approximately 100 nanometers in diameter and their length varies from approximately 100 to 2,000 nanometers in...
membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
of the differentiated enterocyte
Enterocyte
Enterocytes, or intestinal absorptive cells, are simple columnar epithelial cells found in the small intestines and colon. A glycocalyx surface coat contains digestive enzymes. Microvilli on the apical surface increase surface area for the digestion and transport of molecules from the intestinal...
s lining the villi of the small intestine
Small intestine
The small intestine is the part of the gastrointestinal tract following the stomach and followed by the large intestine, and is where much of the digestion and absorption of food takes place. In invertebrates such as worms, the terms "gastrointestinal tract" and "large intestine" are often used to...
. In humans, lactase is encoded by the LCT gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
.
Lactase is essential for digestive hydrolysis of lactose in milk. Deficiency of the enzyme causes lactose intolerance
Lactose intolerance
Lactose intolerance, also called lactase deficiency or hypolactasia, is the inability to digest and metabolize lactose, a sugar found in milk...
.
The optimum temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
for lactase is about 77 °F (25 °C) for its activity and has an optimum pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
of 6.
Function and mechanism
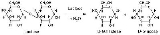
Lactase can hydrolyze a variety of substrates. While it is most notably a member of the β-galactosidase enzymatic class, lactase also has glucosidase and glycosylceramidase activity. In metabolism, the β-glycosidic bond in D-lactose is hydrolyzed to form D-galactose and D-glucose, which can be absorbed through the intestinal walls and into the bloodstream. The overall reaction that lactase catalyzes is C12H22O11 + H2O → C6H12O6 + C6H12O6 + heat. Lactase also catalyzes the conversion of phlorizinPhlorizin
Phlorizin is a toxic 2'-glucoside of phloretin. It belongs to the group of dihydrochalcones, a type of flavonoids. It is a competitive inhibitor of renal glucose transport.Phlorizin is naturally occurring in some plants...
to phloretin
Phloretin
Phloretin is a dihydrochalcone, a type of natural phenols. It is the phloroglucin ester of paraoxyhydratropic acid. It can be found in apple tree leaves.-Metabolism:Phloretin hydrolase uses phloretin and water to produce phloretate and phloroglucinol....
and glucose.
The catalytic mechanism of D-lactose hydrolysis retains the substrate anomeric configuration in the products. While the details of the mechanism are uncertain, the stereochemical retention is achieved through a double displacement reaction. Studies of E. coli lactase have proposed that hydrolysis is initiated when a glutamate nucleophile on the enzyme attacks from the axial side of the galactosyl carbon in the β-glycosidic bond. The removal of the D-glucose leaving group may be facilitated by Mg-dependent acid catalysis. The enzyme is liberated from the α-galactosyl moiety upon equatorial nucleophilic attack by water, which produces D-galactose.
Substrate modification studies have demonstrated that the 3’-OH and 2’-OH moieties on the galactopyranose ring are essential for enzymatic recognition and hydrolysis. The 3’-hydroxy group is involved in initial binding to the substrate while the 2’- group is not necessary for recognition but needed in subsequent steps. This is demonstrated by the fact that a 2-deoxy analog is an effective competitive inhibitor (Ki = 10mM). Elimination of specific hydroxyl groups on the glucopyranose moiety does not completely eliminate catalysis.
Structure and biosynthesis
Pre-pro-lactase, the primary translation product, has a single polypeptide primary structure consisting of 1927 amino acids. It can be divided into five domains: (i) a 19 amino acid cleaved signal sequence; (ii) a large prosequence domain that is not present in mature lactase; (iii) the mature lactase segment; (iv) a membrane spanning hydrophobic anchor; and (v) a short hydrophilic carboxyl terminus. The signal sequence is cleaved in the endoplasmic reticulum, and the resulting 215 kDa pro-LPH is sent to the Golgi, where it is heavily glycosylated and proteolytically processed to its mature form. The prodomain has been shown to act as an intramolecular chaperone in the ER, preventing trypsin cleavage and allowing LPH to adopt the necessary 3-D structure to be transported to the Golgi apparatus.Mature human lactase consists of a single 160 kDa polypeptide chain that localizes to the brush border membrane of intestinal epithelial cells. It is oriented with the N-terminus outside the cell and the C-terminus in the cytosol. LPH contains two catalytic glutamic acid sites. In the human enzyme, the lactase activity has been connected to Glu-1749 while Glu-1273 is the site of phlorizin hydrolase function.
Genetic expression and regulation
Lactase is encoded by a single genetic locus on chromosome 2. It is expressed exclusively by mammalian small intestine enterocytes and in very low levels in the colon during fetal development. Humans are born with high levels of lactase expression. In most of the world’s population, lactase transcription is down-regulated after weaning, resulting in diminished lactase expression in the small intestine. Diminished lactase expression causes the common symptoms of adult-type hypolactasia, or lactose intolerance.Some population segments exhibit lactase persistence resulting from a mutation that is postulated to have occurred 5000-10,000 years ago, coinciding with the rise of cattle domestication. This mutation has allowed almost half of the world’s population to metabolize lactose without symptoms. Studies have linked the occurrence of lactase persistence to two different single-nucleotide polymorphisms about 14 and 22 kilobases upstream of the 5’-end of the LPH gene. Both mutations, C→T at position -13910 and T→ A at position -22018, have been independently linked to lactase persistence.
The lactase promoter is 150 base pairs long and is located just upstream of the site of transcription initiation. The sequence is highly conserved in mammals, suggesting that critical cis-transcriptional regulators are located nearby. Cdx-2, HNF-1α, and GATA have been identified as transcription factors. Studies of hypolactasia onset have demonstrated that despite polymorphisms there is little difference in lactase expression in infants, showing that the mutations become increasingly relevant during development. Ιt is hypothesized that developmentally-regulated DNA-binding proteins down-regulate transcription or destabilize mRNA transcripts, causing decreased LPH expression after weaning.
Industrial use
Lactase produced commercially can be extracted both from yeastYeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
s such as Kluyveromyces fragilis and Kluyveromyces lactis
Kluyveromyces lactis
Kluyveromyces lactis is a Kluyveromyces yeast commonly used for genetic studies and industrial applications. Its name comes from the ability to assimilate lactose and convert it into lactic acid.- Use :...
and from fungi
Fungus
A fungus is a member of a large group of eukaryotic organisms that includes microorganisms such as yeasts and molds , as well as the more familiar mushrooms. These organisms are classified as a kingdom, Fungi, which is separate from plants, animals, and bacteria...
, such as Aspergillus niger
Aspergillus niger
Aspergillus niger is a fungus and one of the most common species of the genus Aspergillus. It causes a disease called black mold on certain fruits and vegetables such as grapes, onions, and peanuts, and is a common contaminant of food...
and Aspergillus oryzae
Aspergillus oryzae
Aspergillus oryzae is a filamentous fungus . It is used in Chinese and Japanese cuisine to ferment soybeans. It is also used to saccharify rice, other grains, and potatoes in the making of alcoholic beverages such as huangjiu, sake, and shōchū...
. Its primary commercial use is to break down lactose in milk to make it suitable for people with lactose intolerance
Lactose intolerance
Lactose intolerance, also called lactase deficiency or hypolactasia, is the inability to digest and metabolize lactose, a sugar found in milk...
. However, the U.S. Food and Drug Administration (FDA) has not formally evaluated the effectiveness of these products. Lactase is also used in the manufacture of ice cream
Ice cream
Ice cream is a frozen dessert usually made from dairy products, such as milk and cream, and often combined with fruits or other ingredients and flavours. Most varieties contain sugar, although some are made with other sweeteners...
. Because glucose and galactose are sweeter than lactose, lactase produces a sweeter ice cream. Lactose also crystallizes at the low temperatures of ice cream; however, its constituent products, glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and galactose
Galactose
Galactose , sometimes abbreviated Gal, is a type of sugar that is less sweet than glucose. It is a C-4 epimer of glucose....
, remain dissolved and contribute to a smoother texture. Lactase is used in the conversion of whey
Whey
Whey or Milk Serum is the liquid remaining after milk has been curdled and strained. It is a by-product of the manufacture of cheese or casein and has several commercial uses. Sweet whey is manufactured during the making of rennet types of hard cheese like cheddar or Swiss cheese...
into syrup.
Lactase is also used to screen for blue white
Blue white screen
The blue-white screen is a screening technique that allows for the detection of successful ligations in vector-based gene cloning. DNA of interest is ligated into a vector. The vector is then transformed into competent cell . The competent cells are grown in the presence of X-gal...
colonies in the MCS
Multiple cloning site
A multiple cloning site , also called a polylinker, is a short segment of DNA which contains many restriction sites - a standard feature of engineered plasmids. Restriction sites within an MCS are typically unique, occurring only once within a given plasmid. MCSs are commonly used during...
of various plasmid vectors in Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
or other bacteria.
See also
- MCM6MCM6DNA replication licensing factor MCM6 is a protein that in humans is encoded by the MCM6 gene. MCM6 is one of the highly conserved mini-chromosome maintenance proteins that are essential for the initiation of eukaryotic genome replication....
- LactoseLactoseLactose is a disaccharide sugar that is found most notably in milk and is formed from galactose and glucose. Lactose makes up around 2~8% of milk , although the amount varies among species and individuals. It is extracted from sweet or sour whey. The name comes from or , the Latin word for milk,...
- Developmental regulation of lactase expression in mammals

