
Lewis structure
Encyclopedia
Lewis structures are diagrams that show the bonding between atom
s of a molecule
and the lone pairs of electron
s that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compound
s. The Lewis structure was named after Gilbert Newton Lewis, who introduced it in his 1916 article The Atom and the Molecule. They are similar to electron dot diagrams in that the valence electrons in lone pairs are represented as dots, but they also contain lines to represent shared pairs in a chemical bond (single, double, triple, etc.).
Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms.
Although many of the elements react by gaining, losing or sharing electrons until they have achieved a valence shell electron configuration with a full octet (8) of electrons, there are many noteworthy exceptions to the 'octet rule'. Hydrogen(H) conforms instead to a duet rule wherein it fills its first (and outermost) shell with just two electrons or empties it completely. Some compounds like boron trifluoride have incomplete orbitals while other such as sulphur hexafluoride have valence shell with more than eight electrons.
s on each individual atom. Non-valence electrons are not represented in Lewis structures.
The octet rule
states atoms with eight electrons in their valence shell will be stable, regardless of whether these electrons are bonding or nonbonding. The rule applies well to acidic compounds. The 18-Electron rule
is operative on atoms from period 4, which have to achieve 18 electrons to their orbitals and achieve a stable configuration which has the same electron configuration as a Noble gas
. Similarly from period 6, the atoms have to achieve 32 electrons to fill their orbitals.
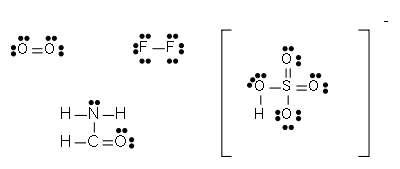 Once the total number of available electrons has been determined, electrons must be placed into the structure. They should be placed initially as lone pairs: one pair of dots for each pair of electrons available. Lone pairs should initially be placed on outer atoms (other than hydrogen) until each outer atom has eight electrons in bonding pairs and lone pairs; extra lone pairs may then be placed on the central atom. When in doubt, lone pairs should be placed on more electronegative atoms first.
Once the total number of available electrons has been determined, electrons must be placed into the structure. They should be placed initially as lone pairs: one pair of dots for each pair of electrons available. Lone pairs should initially be placed on outer atoms (other than hydrogen) until each outer atom has eight electrons in bonding pairs and lone pairs; extra lone pairs may then be placed on the central atom. When in doubt, lone pairs should be placed on more electronegative atoms first.
Once all lone pairs are placed, atoms, especially the central atoms, may not have an octet of electrons. In this case, the atoms must form a double bond; a lone pair of electrons is moved to form a second bond between the two atoms. As the bonding pair is shared between the two atoms, the atom that originally had the lone pair still has an octet; the other atom now has two more electrons in its valence shell.
Aside from organic compounds, only a minority of compounds have an octet of electrons.
Incomplete octets are common for compounds of groups 2 and 13 such as beryllium
, boron
, and aluminium
.
Compounds with more than eight electrons in the Lewis representation of the valence shell of an atom are called hypervalent, and are common for elements of groups 15 to 18, such as phosphorus
, sulfur
, iodine
, and xenon
.
Lewis structures for polyatomic ions may be drawn by the same method. When counting electrons, negative ions should have extra electrons placed in their Lewis structures; positive ions should have fewer electrons than an uncharged molecule.
When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside the brackets.
A simpler method has been proposed for constructing Lewis structures eliminating the need for electron counting: the atoms are drawn showing the valence electrons, bonds are then formed by pairing up valence electrons of the atoms involved in the bond-making process and anions and cations are formed by adding or removing electrons to/from the appropriate atoms.
A trick is to count up valence electrons, then count up the number of electrons needed to complete the octet rule (or with Hydrogen just 2 electrons), then take the difference of these two numbers and your answer is the number of electrons that make up the bonds. The rest of the electrons just go and fill all the other atoms' octets.
is used in the description, comparison and assessment of likely topological
and resonance
structures by determining the apparent electronic charge of each atom within, based upon its electron dot structure assuming exclusive covalency or non-polar bonding. It has uses in determining possible electron re-configuration when referring to reaction mechanism
s, and often results in the same sign as the partial charge
of the atom, with exceptions. In general, the formal charge of an atom can be calculated using the following formula, assuming non-standard definitions for the markup used:

where:
The formal charge of an atom is computed as the difference between the number of valence electrons that a neutral atom would have and the number of electrons that belong to it in the Lewis structure. Electrons in covalent bonds are split equally between the atoms involved in the bond. The total of the formal charges on an ion should be equal to the charge on the ion, and the total of the formal charges on a neutral molecule should be equal to zero.
When this situation occurs, the molecule's Lewis structure is said to be a resonance structure
, and the molecule exists as a resonance hybrid. Each of the different possibilities is superimposed on the others, and the molecule is considered to have a Lewis structure equivalent to an average of these states.
The nitrate ion (NO3-), for instance, must form a double bond between nitrogen and one of the oxygens to satisfy the octet rule for nitrogen. However, because the molecule is symmetrical, it does not matter which of the oxygens forms the double bond. In this case, there are three possible resonance structures. Expressing resonance when drawing Lewis structures may be done either by drawing each of the possible resonance forms and placing double-headed arrows between them or by using dashed lines to represent the partial bonds.
When comparing resonance structures for the same molecule, usually those with the fewest formal charges contribute more to the overall resonance hybrid. When formal charges are necessary, resonance structures that have negative charges on the more electronegative elements and positive charges on the less electronegative elements are favored.
The resonance structure should not be interpreted to indicate that the molecule switches between forms, but that the molecule acts as the average of multiple forms.
ion is NO2−.
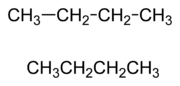
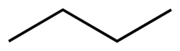
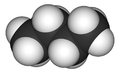 Chemical structures may be written in more compact forms, particularly when showing organic molecules
Chemical structures may be written in more compact forms, particularly when showing organic molecules
. In condensed structural formulas, many or even all of the covalent bonds may be left out, with subscripts indicating the number of identical groups attached to a particular atom.
Another shorthand structural diagram is the skeletal formula
(also known as a bond-line formula or carbon skeleton diagram). In skeletal formulae, carbon atoms are not signified by the symbol C but by the vertices
of the lines. Hydrogen atoms bonded to carbon are not shown — they can be inferred by counting the number of bonds to a particular carbon atom — each carbon is assumed to have four bonds in total, so any bonds not shown are, by implication, to hydrogen atoms.
Other diagrams may be more complex than Lewis structures, showing bonds in 3D using various forms such as space filling diagrams.
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s of a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
and the lone pairs of electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compound
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
s. The Lewis structure was named after Gilbert Newton Lewis, who introduced it in his 1916 article The Atom and the Molecule. They are similar to electron dot diagrams in that the valence electrons in lone pairs are represented as dots, but they also contain lines to represent shared pairs in a chemical bond (single, double, triple, etc.).
Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms.
Although many of the elements react by gaining, losing or sharing electrons until they have achieved a valence shell electron configuration with a full octet (8) of electrons, there are many noteworthy exceptions to the 'octet rule'. Hydrogen(H) conforms instead to a duet rule wherein it fills its first (and outermost) shell with just two electrons or empties it completely. Some compounds like boron trifluoride have incomplete orbitals while other such as sulphur hexafluoride have valence shell with more than eight electrons.
Counting electrons
The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electronValence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s on each individual atom. Non-valence electrons are not represented in Lewis structures.
The octet rule
Octet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
states atoms with eight electrons in their valence shell will be stable, regardless of whether these electrons are bonding or nonbonding. The rule applies well to acidic compounds. The 18-Electron rule
18-Electron rule
The 18-electron rule is a rule of thumb used primarily for predicting formulas for stable metal complexes. The rule rests on the fact that valence shells of a transition metal consists of nine valence orbitals, which collectively can accommodate 18 electrons either as nonbinding electron pairs or...
is operative on atoms from period 4, which have to achieve 18 electrons to their orbitals and achieve a stable configuration which has the same electron configuration as a Noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
. Similarly from period 6, the atoms have to achieve 32 electrons to fill their orbitals.
Placing electrons

Once all lone pairs are placed, atoms, especially the central atoms, may not have an octet of electrons. In this case, the atoms must form a double bond; a lone pair of electrons is moved to form a second bond between the two atoms. As the bonding pair is shared between the two atoms, the atom that originally had the lone pair still has an octet; the other atom now has two more electrons in its valence shell.
Aside from organic compounds, only a minority of compounds have an octet of electrons.
Incomplete octets are common for compounds of groups 2 and 13 such as beryllium
Beryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
, boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
, and aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
.
Compounds with more than eight electrons in the Lewis representation of the valence shell of an atom are called hypervalent, and are common for elements of groups 15 to 18, such as phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
, sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
, iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, and xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
.
Lewis structures for polyatomic ions may be drawn by the same method. When counting electrons, negative ions should have extra electrons placed in their Lewis structures; positive ions should have fewer electrons than an uncharged molecule.
When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside the brackets.
A simpler method has been proposed for constructing Lewis structures eliminating the need for electron counting: the atoms are drawn showing the valence electrons, bonds are then formed by pairing up valence electrons of the atoms involved in the bond-making process and anions and cations are formed by adding or removing electrons to/from the appropriate atoms.
A trick is to count up valence electrons, then count up the number of electrons needed to complete the octet rule (or with Hydrogen just 2 electrons), then take the difference of these two numbers and your answer is the number of electrons that make up the bonds. The rest of the electrons just go and fill all the other atoms' octets.
Formal charge
In terms of Lewis structures, formal chargeFormal charge
In chemistry, a formal charge is the charge assigned to an atom in a molecule, assuming that electrons in a chemical bond are shared equally between atoms, regardless of relative electronegativity....
is used in the description, comparison and assessment of likely topological
Topology
Topology is a major area of mathematics concerned with properties that are preserved under continuous deformations of objects, such as deformations that involve stretching, but no tearing or gluing...
and resonance
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
structures by determining the apparent electronic charge of each atom within, based upon its electron dot structure assuming exclusive covalency or non-polar bonding. It has uses in determining possible electron re-configuration when referring to reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
s, and often results in the same sign as the partial charge
Partial charge
A partial charge is a charge with an absolute value of less than one elementary charge unit .-Partial atomic charges:...
of the atom, with exceptions. In general, the formal charge of an atom can be calculated using the following formula, assuming non-standard definitions for the markup used:

where:
-
 is the Formal charge.
is the Formal charge. -
 represents the number of valence electrons in a free atom of the element.
represents the number of valence electrons in a free atom of the element. -
 represents the number of unshared electrons on the atom.
represents the number of unshared electrons on the atom. -
 represents the total number of bonds the atom has with another.
represents the total number of bonds the atom has with another.
The formal charge of an atom is computed as the difference between the number of valence electrons that a neutral atom would have and the number of electrons that belong to it in the Lewis structure. Electrons in covalent bonds are split equally between the atoms involved in the bond. The total of the formal charges on an ion should be equal to the charge on the ion, and the total of the formal charges on a neutral molecule should be equal to zero.
Resonance
For some molecules and ions, it is difficult to determine which lone pairs should be moved to form double or triple bonds. This is sometimes the case when multiple atoms of the same type surround the central atom, and is especially common for polyatomic ions.When this situation occurs, the molecule's Lewis structure is said to be a resonance structure
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
, and the molecule exists as a resonance hybrid. Each of the different possibilities is superimposed on the others, and the molecule is considered to have a Lewis structure equivalent to an average of these states.
The nitrate ion (NO3-), for instance, must form a double bond between nitrogen and one of the oxygens to satisfy the octet rule for nitrogen. However, because the molecule is symmetrical, it does not matter which of the oxygens forms the double bond. In this case, there are three possible resonance structures. Expressing resonance when drawing Lewis structures may be done either by drawing each of the possible resonance forms and placing double-headed arrows between them or by using dashed lines to represent the partial bonds.
When comparing resonance structures for the same molecule, usually those with the fewest formal charges contribute more to the overall resonance hybrid. When formal charges are necessary, resonance structures that have negative charges on the more electronegative elements and positive charges on the less electronegative elements are favored.
The resonance structure should not be interpreted to indicate that the molecule switches between forms, but that the molecule acts as the average of multiple forms.
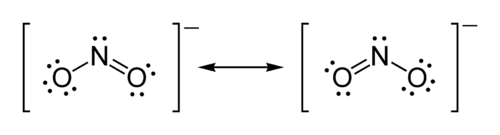
Example: Lewis structure of the nitrite ion
The formula of the nitriteNitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
ion is NO2−.
- Step one: Nitrogen is the least electronegative atom, so it is the central atom by multiple criteria.
- Step two: Count valence electrons. Nitrogen has 5 valence electrons; each oxygen has 6, for a total of (6 × 2) + 5 = 17. The ion has a charge of −1, which indicates an extra electron, so the total number of electrons is 18.
- Step three: Place ion pairs. Each oxygen must be bonded to the nitrogen, which uses four electrons — two in each bond. The 14 remaining electrons should initially be placed as 7 lone pairs. Each oxygen may take a maximum of 3 lone pairs, giving each oxygen 8 electrons including the bonding pair. The seventh lone pair must be placed on the nitrogen atom.
- Step four: Satisfy the octet rule. Both oxygen atoms currently have 8 electrons assigned to them. The nitrogen atom has only 6 electrons assigned to it. One of the lone pairs on an oxygen atom must form a double bond, but either atom will work equally well. We therefore must have a resonance structure.
- Step five: Tie up loose ends. Two Lewis structures must be drawn: one with each oxygen atom double-bonded to the nitrogen atom. The second oxygen atom in each structure will be single-bonded to the nitrogen atom. Place brackets around each structure, and add the charge (−) to the upper right outside the brackets. Draw a double-headed arrow between the two resonance forms.

Alternative formats
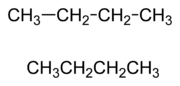


Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
. In condensed structural formulas, many or even all of the covalent bonds may be left out, with subscripts indicating the number of identical groups attached to a particular atom.
Another shorthand structural diagram is the skeletal formula
Skeletal formula
The skeletal formula of an organic compound is a shorthand representation of its molecular structure, developed by the organic chemist, Friedrich August Kekulé von Stradonitz. Skeletal formulae are ubiquitous in organic chemistry, because they are relatively quick and simple to draw. Carbon and...
(also known as a bond-line formula or carbon skeleton diagram). In skeletal formulae, carbon atoms are not signified by the symbol C but by the vertices
Vertex (graph theory)
In graph theory, a vertex or node is the fundamental unit out of which graphs are formed: an undirected graph consists of a set of vertices and a set of edges , while a directed graph consists of a set of vertices and a set of arcs...
of the lines. Hydrogen atoms bonded to carbon are not shown — they can be inferred by counting the number of bonds to a particular carbon atom — each carbon is assumed to have four bonds in total, so any bonds not shown are, by implication, to hydrogen atoms.
Other diagrams may be more complex than Lewis structures, showing bonds in 3D using various forms such as space filling diagrams.
See also
- Valence shell electron pair repulsion theoryVSEPR theoryValence shell electron pair repulsion theory is a model in chemistry used to predict the shape of individual molecules based upon the extent of electron-pair electrostatic repulsion. It is also named Gillespie–Nyholm theory after its two main developers...
- Molecular geometryMolecular geometryMolecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
- Structural formulaStructural formulaThe structural formula of a chemical compound is a graphical representation of the molecular structure, showing how the atoms are arranged. The chemical bonding within the molecule is also shown, either explicitly or implicitly...
- Natural bond orbitalNatural bond orbitalIn quantum chemistry, a natural bond orbital or NBO is a calculated bonding orbital with maximum electron density. The NBOs are one of a sequence of natural localized orbital sets that include "Natural Atomic Orbitals" , "Natural Hybrid Orbitals" , "Natural Bonding Orbitals" and "Natural Localized...

