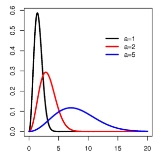
Maxwell–Boltzmann distribution
Encyclopedia
The Maxwell–Boltzmann distribution describes particle speeds in gas
es, where the particles do not constantly interact with each other but move freely between short collision
s. It describes the probability of a particle's speed (the magnitude
of its velocity vector) being near a given value as a function
of the temperature
of the system, the mass of the particle, and that speed value. This probability distribution
is named after James Clerk Maxwell
and Ludwig Boltzmann
.
The Maxwell–Boltzmann distribution is usually thought of as the distribution for molecular speeds, but it can also refer to the distribution for velocities, momenta, and magnitude of the momenta of the molecules, each of which will have a different probability distribution function, all of which are related. Unless otherwise stated, this article will use "Maxwell–Boltzmann distribution" to refer to the distribution of speed. This distribution can be thought of as the magnitude of a 3-dimensional vector whose components are independent and normally distributed with mean 0 and standard deviation
 . If
. If  are distributed as
are distributed as  , then
, then
is distributed as a Maxwell–Boltzmann distribution with parameter . Apart from the scale parameter
. Apart from the scale parameter  , the distribution is identical to the chi distribution with 3 degrees of freedom.
, the distribution is identical to the chi distribution with 3 degrees of freedom.
with negligible quantum effects and at non-relativistic speeds. It forms the basis of the kinetic theory of gases, which explains many fundamental gas properties, including pressure
and diffusion
.
assumed all three directions would behave in the same fashion, but a later derivation by Boltzmann dropped this assumption using kinetic theory
. The Maxwell–Boltzmann distribution (for energies) can now most readily be derived from the Boltzmann distribution
for energies (see also the Maxwell–Boltzmann statistics
of statistical mechanics
):

where:
Note that sometimes the above equation is written without the degeneracy factor gi. In this case the index i will specify an individual state, rather than a set of gi states having the same energy Ei. Because velocity and speed are related to energy, Equation 1 can be used to derive relationships between temperature and the speeds of molecules in a gas. The denominator in this equation is known as the canonical partition function
.
and later described with fewer assumptions by Ludwig Boltzmann
. Instead it is close to Boltzmann's later approach of 1877.
For the case of an "ideal gas" consisting of non-interacting atoms in the ground state, all energy is in the form of kinetic energy, and gi is constant for all i. The relationship between kinetic energy and momentum for massive particles is

where p2 is the square of the momentum vector
p = [px, py, pz]. We may therefore rewrite Equation 1 as:

where Z is the partition function
, corresponding to the denominator in Equation 1. Here m is the molecular mass of the gas, T is the thermodynamic temperature and k is the Boltzmann constant. This distribution of Ni/N is proportional
to the probability density function
fp for finding a molecule with these values of momentum components, so:

The normalizing constant
c, can be determined by recognizing that the probability of a molecule having any momentum must be 1. Therefore the integral of equation 4 over all px, py, and pz must be 1.
It can be shown that:
Substituting Equation 5 into Equation 4 gives:

The distribution is seen to be the product of three independent normally distributed variables ,
,  , and
, and  , with variance
, with variance  . Additionally, it can be seen that the magnitude of momentum will be distributed as a Maxwell–Boltzmann distribution, with
. Additionally, it can be seen that the magnitude of momentum will be distributed as a Maxwell–Boltzmann distribution, with  .
.
The Maxwell–Boltzmann distribution for the momentum (or equally for the velocities) can be obtained more fundamentally using the H-theorem
at equilibrium within the kinetic theory
framework.

Since the energy is proportional to the sum of the squares of the three normally distributed momentum components, this distribution is a gamma distribution and a chi-squared distribution with three degrees of freedom.
By the equipartition theorem
, this energy is evenly distributed among all three degrees of freedom, so that the energy per degree of freedom is distributed as a chi-squared distribution with one degree of freedom:
where is the energy per degree of freedom. At equilibrium, this distribution will hold true for any number of degrees of freedom. For example, if the particles are rigid mass dipoles, they will have three translational degrees of freedom and two additional rotational degrees of freedom. The energy in each degree of freedom will be described according to the above chi-squared distribution with one degree of freedom, and the total energy will be distributed according to a chi-squared distribution with five degrees of freedom. This has implications in the theory of the specific heat of a gas.
is the energy per degree of freedom. At equilibrium, this distribution will hold true for any number of degrees of freedom. For example, if the particles are rigid mass dipoles, they will have three translational degrees of freedom and two additional rotational degrees of freedom. The energy in each degree of freedom will be described according to the above chi-squared distribution with one degree of freedom, and the total energy will be distributed according to a chi-squared distribution with five degrees of freedom. This has implications in the theory of the specific heat of a gas.
The Maxwell–Boltzmann distribution can also be obtained by considering the gas to be a type of quantum gas
.

and using p = mv we get

which is the Maxwell–Boltzmann velocity distribution. The probability of finding a particle with velocity in the infinitesimal element [dvx, dvy, dvz] about velocity v = [vx, vy, vz] is

Like the momentum, this distribution is seen to be the product of three independent normally distributed variables ,
,  , and
, and  , but with variance
, but with variance  . It can also be seen that the Maxwell–Boltzmann velocity distribution for the vector velocity
. It can also be seen that the Maxwell–Boltzmann velocity distribution for the vector velocity
[vx, vy, vz] is the product of the distributions for each of the three directions:

where the distribution for a single direction is

Each component of the velocity vector has a normal distribution with mean and standard deviation
and standard deviation  , so the vector has a 3-dimensional normal distribution, also called a "multinormal" distribution, with mean
, so the vector has a 3-dimensional normal distribution, also called a "multinormal" distribution, with mean  and standard deviation
and standard deviation  .
.
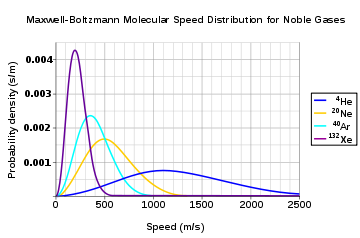 Usually, we are more interested in the speeds of molecules rather than their component velocities. The Maxwell–Boltzmann distribution for the speed follows immediately from the distribution of the velocity vector, above. Note that the speed is
Usually, we are more interested in the speeds of molecules rather than their component velocities. The Maxwell–Boltzmann distribution for the speed follows immediately from the distribution of the velocity vector, above. Note that the speed is

and the increment of volume is

where and
and  are the "course" (azimuth of the velocity vector) and "path angle" (elevation angle of the velocity vector). Integration of the normal probability density function of the velocity, above, over the course (from 0 to
are the "course" (azimuth of the velocity vector) and "path angle" (elevation angle of the velocity vector). Integration of the normal probability density function of the velocity, above, over the course (from 0 to  ) and path angle (from 0 to
) and path angle (from 0 to  ), with substitution of the speed for the sum of the squares of the vector components, yields the probability density function
), with substitution of the speed for the sum of the squares of the vector components, yields the probability density function

for the speed. This equation is simply the Maxwell distribution with distribution parameter .
.
We are often more interested in quantities such as the average speed of the particles rather than the actual distribution. The mean speed, most probable speed (mode), and root-mean-square can be obtained from properties of the Maxwell distribution.
 , where
, where  is the most probable speed. The distribution of relative speeds allows comparison of dissimilar gasses, independent of temperature and molecular weight.
is the most probable speed. The distribution of relative speeds allows comparison of dissimilar gasses, independent of temperature and molecular weight.
The most probable speed, vp, is the speed most likely to be possessed by any molecule (of the same mass m) in the system and corresponds to the maximum value or mode
of f(v). To find it, we calculate df/dv, set it to zero and solve for v:

which yields:

Where R is the gas constant
and M = NA m is the molar mass
of the substance.
For diatomic nitrogen (N2, the primary component of air) at room temperature
(300 K), this gives m/s
m/s
The mean speed is the mathematical average of the speed distribution

The root mean square speed
, vrms is the square root of the average squared speed:

The typical speeds are related as follows:

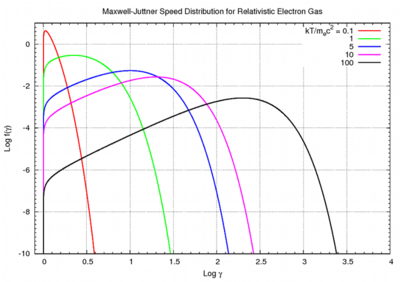 As the gas becomes hotter and kT approaches or exceeds mc2, the probability distribution for
As the gas becomes hotter and kT approaches or exceeds mc2, the probability distribution for  in this relativistic Maxwellian gas is given by the Maxwell–Juttner distribution:
in this relativistic Maxwellian gas is given by the Maxwell–Juttner distribution:

where
 and
and  is the modified Bessel function
is the modified Bessel function
of the second kind.
Alternatively, this can be written in terms of the momentum as

where . The Maxwell–Juttner equation is covariant, but not manifestly so, and the temperature of the gas does not vary with the gross speed of the gas.
. The Maxwell–Juttner equation is covariant, but not manifestly so, and the temperature of the gas does not vary with the gross speed of the gas.
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
es, where the particles do not constantly interact with each other but move freely between short collision
Collision
A collision is an isolated event which two or more moving bodies exert forces on each other for a relatively short time.Although the most common colloquial use of the word "collision" refers to accidents in which two or more objects collide, the scientific use of the word "collision" implies...
s. It describes the probability of a particle's speed (the magnitude
Magnitude (mathematics)
The magnitude of an object in mathematics is its size: a property by which it can be compared as larger or smaller than other objects of the same kind; in technical terms, an ordering of the class of objects to which it belongs....
of its velocity vector) being near a given value as a function
Function (mathematics)
In mathematics, a function associates one quantity, the argument of the function, also known as the input, with another quantity, the value of the function, also known as the output. A function assigns exactly one output to each input. The argument and the value may be real numbers, but they can...
of the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
of the system, the mass of the particle, and that speed value. This probability distribution
Probability distribution
In probability theory, a probability mass, probability density, or probability distribution is a function that describes the probability of a random variable taking certain values....
is named after James Clerk Maxwell
James Clerk Maxwell
James Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
and Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
.
The Maxwell–Boltzmann distribution is usually thought of as the distribution for molecular speeds, but it can also refer to the distribution for velocities, momenta, and magnitude of the momenta of the molecules, each of which will have a different probability distribution function, all of which are related. Unless otherwise stated, this article will use "Maxwell–Boltzmann distribution" to refer to the distribution of speed. This distribution can be thought of as the magnitude of a 3-dimensional vector whose components are independent and normally distributed with mean 0 and standard deviation
Standard deviation
Standard deviation is a widely used measure of variability or diversity used in statistics and probability theory. It shows how much variation or "dispersion" there is from the average...
 . If
. If  are distributed as
are distributed as  , then
, then
is distributed as a Maxwell–Boltzmann distribution with parameter
 . Apart from the scale parameter
. Apart from the scale parameter  , the distribution is identical to the chi distribution with 3 degrees of freedom.
, the distribution is identical to the chi distribution with 3 degrees of freedom.Physical applications of the Maxwell–Boltzmann distribution
The Maxwell–Boltzmann distribution applies to ideal gases close to thermodynamic equilibriumThermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
with negligible quantum effects and at non-relativistic speeds. It forms the basis of the kinetic theory of gases, which explains many fundamental gas properties, including pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
.
Derivation
The original derivation by MaxwellJames Clerk Maxwell
James Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
assumed all three directions would behave in the same fashion, but a later derivation by Boltzmann dropped this assumption using kinetic theory
Kinetic theory
The kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
. The Maxwell–Boltzmann distribution (for energies) can now most readily be derived from the Boltzmann distribution
Boltzmann distribution
In chemistry, physics, and mathematics, the Boltzmann distribution is a certain distribution function or probability measure for the distribution of the states of a system. It underpins the concept of the canonical ensemble, providing its underlying distribution...
for energies (see also the Maxwell–Boltzmann statistics
Maxwell–Boltzmann statistics
In statistical mechanics, Maxwell–Boltzmann statistics describes the statistical distribution of material particles over various energy states in thermal equilibrium, when the temperature is high enough and density is low enough to render quantum effects negligible.The expected number of particles...
of statistical mechanics
Statistical mechanics
Statistical mechanics or statistical thermodynamicsThe terms statistical mechanics and statistical thermodynamics are used interchangeably...
):

where:
- i is the microstateMicrostate (statistical mechanics)In statistical mechanics, a microstate is a specific microscopic configuration of a thermodynamic system that the system may occupy with a certain probability in the course of its thermal fluctuations...
(indicating one configuration particle quantum states - see partition functionPartition function (statistical mechanics)Partition functions describe the statistical properties of a system in thermodynamic equilibrium. It is a function of temperature and other parameters, such as the volume enclosing a gas...
). - Ei is the energy level of microstate i.
- T is the equilibrium temperature of the system.
- gi is the degeneracy factor, or number of degenerateDegenerate energy levelIn physics, two or more different quantum states are said to be degenerate if they are all at the same energy level. Statistically this means that they are all equally probable of being filled, and in Quantum Mechanics it is represented mathematically by the Hamiltonian for the system having more...
microstates which have the same energy level - k is the Boltzmann constant.
- Ni is the number of molecules at equilibrium temperature T, in a state i which has energy Ei and degeneracy gi.
- N is the total number of molecules in the system.
Note that sometimes the above equation is written without the degeneracy factor gi. In this case the index i will specify an individual state, rather than a set of gi states having the same energy Ei. Because velocity and speed are related to energy, Equation 1 can be used to derive relationships between temperature and the speeds of molecules in a gas. The denominator in this equation is known as the canonical partition function
Partition function (statistical mechanics)
Partition functions describe the statistical properties of a system in thermodynamic equilibrium. It is a function of temperature and other parameters, such as the volume enclosing a gas...
.
Distribution for the momentum vector
What follows is a derivation wildly different from the derivation described by James Clerk MaxwellJames Clerk Maxwell
James Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
and later described with fewer assumptions by Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
. Instead it is close to Boltzmann's later approach of 1877.
For the case of an "ideal gas" consisting of non-interacting atoms in the ground state, all energy is in the form of kinetic energy, and gi is constant for all i. The relationship between kinetic energy and momentum for massive particles is

where p2 is the square of the momentum vector
p = [px, py, pz]. We may therefore rewrite Equation 1 as:

where Z is the partition function
Partition function (statistical mechanics)
Partition functions describe the statistical properties of a system in thermodynamic equilibrium. It is a function of temperature and other parameters, such as the volume enclosing a gas...
, corresponding to the denominator in Equation 1. Here m is the molecular mass of the gas, T is the thermodynamic temperature and k is the Boltzmann constant. This distribution of Ni/N is proportional
Proportionality (mathematics)
In mathematics, two variable quantities are proportional if one of them is always the product of the other and a constant quantity, called the coefficient of proportionality or proportionality constant. In other words, are proportional if the ratio \tfrac yx is constant. We also say that one...
to the probability density function
Probability density function
In probability theory, a probability density function , or density of a continuous random variable is a function that describes the relative likelihood for this random variable to occur at a given point. The probability for the random variable to fall within a particular region is given by the...
fp for finding a molecule with these values of momentum components, so:

The normalizing constant
Normalizing constant
The concept of a normalizing constant arises in probability theory and a variety of other areas of mathematics.-Definition and examples:In probability theory, a normalizing constant is a constant by which an everywhere non-negative function must be multiplied so the area under its graph is 1, e.g.,...
c, can be determined by recognizing that the probability of a molecule having any momentum must be 1. Therefore the integral of equation 4 over all px, py, and pz must be 1.
It can be shown that:

Substituting Equation 5 into Equation 4 gives:

The distribution is seen to be the product of three independent normally distributed variables
 ,
,  , and
, and  , with variance
, with variance  . Additionally, it can be seen that the magnitude of momentum will be distributed as a Maxwell–Boltzmann distribution, with
. Additionally, it can be seen that the magnitude of momentum will be distributed as a Maxwell–Boltzmann distribution, with  .
.The Maxwell–Boltzmann distribution for the momentum (or equally for the velocities) can be obtained more fundamentally using the H-theorem
H-theorem
In Classical Statistical Mechanics, the H-theorem, introduced by Ludwig Boltzmann in 1872, describes the increase in the entropy of an ideal gas in an irreversible process. H-theorem follows from considerations of Boltzmann's equation...
at equilibrium within the kinetic theory
Kinetic theory
The kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
framework.
Distribution for the energy
Using p² = 2mE, and the distribution function for the magnitude of the momentum (see below), we get the energy distribution:
Since the energy is proportional to the sum of the squares of the three normally distributed momentum components, this distribution is a gamma distribution and a chi-squared distribution with three degrees of freedom.
By the equipartition theorem
Equipartition theorem
In classical statistical mechanics, the equipartition theorem is a general formula that relates the temperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition...
, this energy is evenly distributed among all three degrees of freedom, so that the energy per degree of freedom is distributed as a chi-squared distribution with one degree of freedom:

where
 is the energy per degree of freedom. At equilibrium, this distribution will hold true for any number of degrees of freedom. For example, if the particles are rigid mass dipoles, they will have three translational degrees of freedom and two additional rotational degrees of freedom. The energy in each degree of freedom will be described according to the above chi-squared distribution with one degree of freedom, and the total energy will be distributed according to a chi-squared distribution with five degrees of freedom. This has implications in the theory of the specific heat of a gas.
is the energy per degree of freedom. At equilibrium, this distribution will hold true for any number of degrees of freedom. For example, if the particles are rigid mass dipoles, they will have three translational degrees of freedom and two additional rotational degrees of freedom. The energy in each degree of freedom will be described according to the above chi-squared distribution with one degree of freedom, and the total energy will be distributed according to a chi-squared distribution with five degrees of freedom. This has implications in the theory of the specific heat of a gas.The Maxwell–Boltzmann distribution can also be obtained by considering the gas to be a type of quantum gas
Gas in a box
In quantum mechanics, the results of the quantum particle in a box can be used to look at the equilibrium situation for a quantum ideal gas in a box which is a box containing a large number of molecules which do not interact with each other except for instantaneous thermalizing collisions...
.
Distribution for the velocity vector
Recognizing that the velocity probability density fv is proportional to the momentum probability density function by
and using p = mv we get

which is the Maxwell–Boltzmann velocity distribution. The probability of finding a particle with velocity in the infinitesimal element [dvx, dvy, dvz] about velocity v = [vx, vy, vz] is

Like the momentum, this distribution is seen to be the product of three independent normally distributed variables
 ,
,  , and
, and  , but with variance
, but with variance  . It can also be seen that the Maxwell–Boltzmann velocity distribution for the vector velocity
. It can also be seen that the Maxwell–Boltzmann velocity distribution for the vector velocity[vx, vy, vz] is the product of the distributions for each of the three directions:

where the distribution for a single direction is

Each component of the velocity vector has a normal distribution with mean
 and standard deviation
and standard deviation  , so the vector has a 3-dimensional normal distribution, also called a "multinormal" distribution, with mean
, so the vector has a 3-dimensional normal distribution, also called a "multinormal" distribution, with mean  and standard deviation
and standard deviation  .
.Distribution for the speed


and the increment of volume is

where
 and
and  are the "course" (azimuth of the velocity vector) and "path angle" (elevation angle of the velocity vector). Integration of the normal probability density function of the velocity, above, over the course (from 0 to
are the "course" (azimuth of the velocity vector) and "path angle" (elevation angle of the velocity vector). Integration of the normal probability density function of the velocity, above, over the course (from 0 to  ) and path angle (from 0 to
) and path angle (from 0 to  ), with substitution of the speed for the sum of the squares of the vector components, yields the probability density function
), with substitution of the speed for the sum of the squares of the vector components, yields the probability density function
for the speed. This equation is simply the Maxwell distribution with distribution parameter
 .
.We are often more interested in quantities such as the average speed of the particles rather than the actual distribution. The mean speed, most probable speed (mode), and root-mean-square can be obtained from properties of the Maxwell distribution.
Distribution for relative speed
Relative speed is defined as , where
, where  is the most probable speed. The distribution of relative speeds allows comparison of dissimilar gasses, independent of temperature and molecular weight.
is the most probable speed. The distribution of relative speeds allows comparison of dissimilar gasses, independent of temperature and molecular weight.Typical speeds
Although the above equation gives the distribution for the speed or, in other words, the fraction of time the molecule has a particular speed, we are often more interested in quantities such as the average speed rather than the whole distribution.The most probable speed, vp, is the speed most likely to be possessed by any molecule (of the same mass m) in the system and corresponds to the maximum value or mode
Mode (statistics)
In statistics, the mode is the value that occurs most frequently in a data set or a probability distribution. In some fields, notably education, sample data are often called scores, and the sample mode is known as the modal score....
of f(v). To find it, we calculate df/dv, set it to zero and solve for v:

which yields:

Where R is the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and M = NA m is the molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
of the substance.
For diatomic nitrogen (N2, the primary component of air) at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
(300 K), this gives
 m/s
m/sThe mean speed is the mathematical average of the speed distribution

The root mean square speed
Root mean square speed
Root-mean-square speed is the measure of the speed of particles in a gas that is most convenient for problem solving within the kinetic theory of gases. It is defined as the square root of the average velocity-squared of the molecules in a gas...
, vrms is the square root of the average squared speed:

The typical speeds are related as follows:

Distribution for relativistic speeds

 in this relativistic Maxwellian gas is given by the Maxwell–Juttner distribution:
in this relativistic Maxwellian gas is given by the Maxwell–Juttner distribution:
where

 and
and  is the modified Bessel function
is the modified Bessel functionBessel function
In mathematics, Bessel functions, first defined by the mathematician Daniel Bernoulli and generalized by Friedrich Bessel, are canonical solutions y of Bessel's differential equation:...
of the second kind.
Alternatively, this can be written in terms of the momentum as

where
 . The Maxwell–Juttner equation is covariant, but not manifestly so, and the temperature of the gas does not vary with the gross speed of the gas.
. The Maxwell–Juttner equation is covariant, but not manifestly so, and the temperature of the gas does not vary with the gross speed of the gas.See also
- Maxwell–Boltzmann statisticsMaxwell–Boltzmann statisticsIn statistical mechanics, Maxwell–Boltzmann statistics describes the statistical distribution of material particles over various energy states in thermal equilibrium, when the temperature is high enough and density is low enough to render quantum effects negligible.The expected number of particles...
- Boltzmann distributionBoltzmann distributionIn chemistry, physics, and mathematics, the Boltzmann distribution is a certain distribution function or probability measure for the distribution of the states of a system. It underpins the concept of the canonical ensemble, providing its underlying distribution...
- Maxwell speed distributionMaxwell Speed DistributionClassically, an ideal gas' molecules bounce around with somewhat arbitrary velocities, never interacting with each other. In reality, however, an ideal gas is subjected to intermolecular forces. It is to be noted that the aforementioned classical treatment of an ideal gas is only useful when...
- Boltzmann factorBoltzmann factorIn physics, the Boltzmann factor is a weighting factor that determines the relative probability of a particle to be in a state i in a multi-state system in thermodynamic equilibrium at temperature T...
- Rayleigh distribution
- Ideal gas lawIdeal gas lawThe ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
- James Clerk MaxwellJames Clerk MaxwellJames Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
- Ludwig Eduard Boltzmann
- Kinetic theoryKinetic theoryThe kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
External links
- "The Maxwell Speed Distribution" from The Wolfram Demonstrations Project at MathworldMathWorldMathWorld is an online mathematics reference work, created and largely written by Eric W. Weisstein. It is sponsored by and licensed to Wolfram Research, Inc. and was partially funded by the National Science Foundation's National Science Digital Library grant to the University of Illinois at...

