
Organozinc compound
Encyclopedia
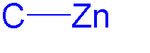
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
contain carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
to zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.
The first organozinc compound ever prepared, diethylzinc
Diethylzinc
Diethylzinc 2Zn, or DEZn, is a highly pyrophoric organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry and available commercially as a solution in hexanes, heptane, or toluene.-Synthesis:Edward Frankland first...
(by Edward Frankland
Edward Frankland
Sir Edward Frankland, KCB, FRS was a chemist, one of the foremost of his day. He was an expert in water quality and analysis, and originated the concept of combining power, or valence, in chemistry. He was also one of the originators of organometallic chemistry.-Biography:Edward Frankland was born...
in 1849), was also the first compound discovered with a metal-to-carbon sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
. Many organozinc compounds are pyrophoric and therefore difficult to handle. Organozinc compounds in general are sensitive to oxidation, dissolve in a wide variety of solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s where protic solvents cause decomposition. In many reactions they are prepared in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
, not isolated and reacted further. All reactions require a protective gas (nitrogen or argon) blanket.
The most common oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
is +2. The three main classes of organozincs are: organozinc halides R-Zn-X with X a halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
atom, diorganozincs R-Zn-R with R an alkyl or aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
group and lithium zincates or magnesium zincates M+R3Zn- with M lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
or magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
.
The carbon zinc bond is polarized towards carbon due to the differences in electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
(carbon:2.55 and zinc: 1.65). Diorganozincs are always monomeric, the organozinc halides form aggregates through halogen bridges very much like Grignard reagents and also like Grignards they display a Schlenk equilibrium
Schlenk equilibrium
The Schlenk equilibrium is a chemical equilibrium named after its discoverer Wilhelm Schlenk taking place in solutions of Grignard reagents.The process described is an equilibrium between two equivalents of an alkyl or aryl magnesium halide on the left of the equation and on the right side, one...
.
Synthesis
Several general methods exist for the generation of organozinc compounds.- Oxidative additionOxidative additionOxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
. The original diethylzinc synthesis by Frankland was an oxidative addition of iodoethane to zinc metal with hydrogen gas as a "protective" blanket (this reaction is called the Frankland synthesis). The reactivity of zinc metal is increased in so-called Rieke zincRieke metalsRieke metals are highly reactive metal powders prepared by the methods developed by Reuben D. Rieke. Rieke metals are highly reactive because they have high surface area and lack surface oxides which retard reaction.-Preparation:...
obtained by reduction of zinc chlorideZinc chlorideZinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
by potassiumPotassiumPotassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
metal.
- 2RI + 2Zn → ZnR2 + ZnI2
- Halogen zinc exchange. Two main halogen zinc exchange reactions are iodine zinc exchange and boron zinc exchange. The first step in the second procedure is hydroboration of an alkene:

- TransmetalationTransmetalationTransmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
. In a typical transmetalation, diphenylmercuryDiphenylmercuryDiphenylmercury is a colourless, crystalline organomercury compound with the chemical formula C12H10Hg. It can be synthesised by the reaction of a 2:1 molar ratio of mercury chloride and methyltriphenyltin in ethanol...
reacts with zinc metal to diphenylzincDiphenylzincDiphenylzinc is an organozinc compound. It is commonly used as the synthetic equivalent of a a Ph− synthon. Solvent-free diphenylzinc exists as dimeric PhZn2ZnPh molecules in solid state....
and metallic mercuryMercury (element)Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
in diethyl etherDiethyl etherDiethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
. Unfortunately, this reaction is slow, taking two weeks. - Organozinc compounds can be obtained directly from zinc metal:
- Transmetalation

- In this method zinc is activated by the action of 1,2-dibromoethane1,2-Dibromoethane1,2-Dibromoethane, also known as ethylene dibromide , is the chemical compound with the formula BrCH2CH2Br. Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly a synthetic...
and trimethylsilyl chlorideTrimethylsilyl chlorideTrimethylsilyl chloride, also known as chlorotrimethylsilane is a silyl halide, with a variety of different uses in chemistry. It has the formula 3SiCl, and under standard conditions it is a colourless liquid, which is stable in the absence of water...
. A key ingredient is lithium chlorideLithium chlorideLithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound, although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents and its hygroscopic...
which quickly forms a soluble adduct with the organozinc compound thus removing it from the metal surface.
Reactions
In many of their reactions organozincs appear as intermediates.- In the Frankland-Duppa Reaction (1863) an oxalateOxalateOxalate , is the dianion with formula C2O42− also written 22−. Either name is often used for derivatives, such as disodium oxalate, 2C2O42−, or an ester of oxalic acid Oxalate (IUPAC: ethanedioate), is the dianion with formula C2O42− also written (COO)22−. Either...
ester (ROCOCOOR) reacts with an alkyl halide R'X, zinc and hydrochloric acidHydrochloric acidHydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
to the α-hydroxycarboxylic esters RR'COHCOOR - The Reformatskii reactionReformatskii reactionThe Reformatsky reaction is an organic reaction which condenses aldehydes , 1, with α-halo esters, 2, using a metallic zinc to form β-hydroxy-esters, 3...
converts α-halo-esters and aldehydes to β-hydroxy-esters also through an intermediate organozinc halide. - In the Simmons-Smith reactionSimmons-Smith reactionThe Simmons–Smith reaction is an organic cheletropic reaction in which a carbenoid reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and R. D. Smith...
the carbenoid (iodomethyl)zinc iodide reacts with alkens to cyclopropanes - Reactions of zinc metal acetylides
- Addition reactionAddition reactionAn addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
of organozinc compounds to carbonylCarbonylIn organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds. The Barbier reactionBarbier reactionThe Barbier reaction is an organic reaction between an alkyl halide and a carbonyl group as an electrophilic substrate in the presence of magnesium, aluminium, zinc, indium, tin or its salts. The reaction product is a primary, secondary or tertiary alcohol...
(1899) is the zinc equivalent of the magnesium Grignard reactionGrignard reactionThe Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
and actually the older and the more tolerant of the two. In presence of just about any water the formation of the organomagnesium halide will fail whereas the Barbier reaction can even take place in water. On the downside organozincs are much less nucleophilic than Grignards. Among the Group 12 elementGroup 12 elementA group 12 element is one of the elements in group 12 in the periodic table. This includes zinc , cadmium and mercury . The further inclusion of copernicium in group 12 is supported by recent experiments on individual Cn atoms...
s zinc is the most reactive. Commercially available diorganozinc compounds are dimethylzincDimethylzincDimethylzinc, also known as Zinc methyl, DMZ, or DMZn is a colorless mobile liquid Zn2, formed by the action of methyl iodide on zinc at elevated temperature or on zinc sodium alloy....
, diethylzincDiethylzincDiethylzinc 2Zn, or DEZn, is a highly pyrophoric organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry and available commercially as a solution in hexanes, heptane, or toluene.-Synthesis:Edward Frankland first...
and diphenylzinc. These reagents are expensive and difficult to handle. In one study the active organozinc compound is obtained from much cheaper organobromineOrganobromine compoundOrganobromine compounds are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application is the use of polybrominated diphenyl ethers as fire-retardants. A variety of minor organobromine compounds are found in...
precursors:
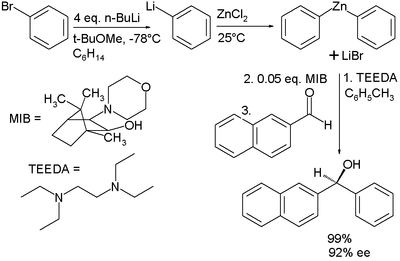
- The Negishi couplingNegishi couplingThe Negishi coupling is a cross coupling reaction in organic chemistry involving an organozinc compound, an organic halide and a nickel or palladium catalyst creating a new carbon-carbon covalent bond:* The halide X can be chloride, bromine or iodine but also a triflate or acetyloxy group with as...
is an important reaction for the formation of new carbon carbon bonds between unsaturated carbon atoms in alkenes, arenes and alkynes. The catalysts are nickel and palladium. A key step in the catalytic cycleCatalytic cycleA catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
is a transmetalationTransmetalationTransmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
in which a zinc halide exchanges its organic substituent for another halogen with the palladium (nickel) metal center. The Fukuyama couplingFukuyama couplingThe Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998...
is another coupling reaction but this one with a thioester as reactant forming a ketone.
- The Negishi coupling
Organozincates
The first ever organozinc ate complexAte complex
An ate complex in chemistry is a salt formed by reaction of a Lewis acid with a base whereby the central atom increases its valence . Often in chemical nomenclature the phrase ate is suffixed to the element in question. For example, the ate complex of a boron compound is called a borate...
(organozincate) was discovered in 1858 by James Alfred Wanklyn
James Alfred Wanklyn
James Alfred Wanklyn was a nineteenth-century English chemist who is remember today chiefly for his "ammonia method" of determining water quality and for his fierce arguments with those, such as Edward Frankland, who opposed him over matters related to water analysis. Wanklyn was born in...
, an assistant to Frankland and concerned the reaction of elemental sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
with diethylzinc
Diethylzinc
Diethylzinc 2Zn, or DEZn, is a highly pyrophoric organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry and available commercially as a solution in hexanes, heptane, or toluene.-Synthesis:Edward Frankland first...
:
- 2Na + 3 Et2Zn -> 2Et3Zn-Na+ + Zn
In 2007 it was reported that by tweaking the reaction conditions the zincate proceeds to react to sodium hydridoethylzincate(II) (with hydrogen atoms as bridging ligand
Bridging ligand
A bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are...
s) as a result of beta-hydride elimination
Beta-hydride elimination
Beta-hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the beta carbon. For instance butyl groups can undergo this reaction but methyl groups cannot...
of one of the ethyl groups:
Organozinc(I) compounds
Low valent organozinc compounds having a Zn-Zn bond are also known. The first such compound DecamethyldizincoceneDecamethyldizincocene
Decamethyldizincocene is an organozinc compound with the formula [Zn22]. It is an unusual example of a compound with a Zn-Zn bond. Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the presence of oxygen and reacts with water...
was reported in 2004
See also
- Compounds of zincCompounds of zincCompounds of zinc are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc having much chemical...
- Compounds of carbon with other elements in the periodic table:


