
Paracetamol toxicity
Encyclopedia
Paracetamol toxicity is caused by excessive use or overdose of the analgesic
drug paracetamol
(called acetaminophen in North America). Mainly causing liver injury, paracetamol toxicity is one of the most common causes of poisoning worldwide. In the United States
and the United Kingdom
it is the most common cause of acute liver failure.
Many individuals with paracetamol toxicity may have no symptoms at all in the first 24 hours following overdose. Others may initially have nonspecific complaints such as vague abdominal pain
and nausea
. With progressive disease, signs of liver failure may develop; these include low blood sugar
, low blood pH
, easy bleeding
, and hepatic encephalopathy
. Some will spontaneously resolve, although untreated cases may result in death.
Damage to the liver, or hepatotoxicity
, results not from paracetamol itself, but from one of its metabolite
s, N-acetyl-p-benzoquinoneimine
(NAPQI). NAPQI depletes the liver's natural antioxidant
glutathione
and directly damages cells in the liver, leading to liver failure. Risk factors for toxicity include excessive chronic alcohol
intake, fasting
or anorexia nervosa
, and the use of certain drugs such as isoniazid
.
Treatment is aimed at removing the paracetamol from the body and replacing glutathione. Activated charcoal can be used to decrease absorption of paracetamol if the patient presents for treatment soon after the overdose; the antidote acetylcysteine acts as a precursor for glutathione, helping the body regenerate enough to prevent damage to the liver. A liver transplant is often required if damage to the liver becomes severe. Patients treated early have a good prognosis, whereas patients that develop major liver abnormalities typically have a poor outcome. Efforts to prevent paracetamol overdose include limiting individual sales of the drug and combining paracetamol with methionine
, which is converted into glutathione in the liver.
in their liver
to typically
about three times the normal value. It is unlikely that this dose would lead to liver failure
. Studies have shown significant hepatotoxicity is uncommon in patients who have taken greater than normal doses over 3 to 4 days. In adults, a dose of 6 grams a day over the preceding 48 hours could potentially lead to toxicity, while in children acute doses above 200 mg/kg could potentially cause toxicity. Acute paracetamol overdose in children rarely causes illness or death, and it is very uncommon for children to have levels that require treatment, with chronic larger-than-normal doses being the major cause of toxicity in children. Intravenous doses should be smaller than those taken orally, all other things being equal.
In rare individuals, paracetamol toxicity can result from normal use. This may be due to individual ("idiosyncratic") differences in the expression and activity of certain enzymes in one of the metabolic pathway
s that handle paracetamol (see paracetamol's metabolism).
, vomiting
, pallor
, and sweating
. However, patients often have no specific symptoms or only mild symptoms in the first 24 hours of poisoning. Rarely, after massive overdoses, patients may develop symptoms of metabolic acidosis
and coma
early in the course of poisoning.
The second phase occurs between 24 and 72 hours following overdose and consists of signs of increasing liver damage. In general, damage occurs in hepatocyte
s as they metabolize the paracetamol. The individual may experience right-upper-quadrant pain. The increasing liver damage also alters biochemical markers of liver function; International normalized ratio (INR) and the hepatic transaminase
s alanine transaminase
and aspartate transaminase
rise to abnormal levels. Acute kidney failure may also occur during this phase, typically caused by either hepatorenal syndrome
or multiple organ dysfunction syndrome
. In some cases, acute kidney failure may be the primary clinical manifestation of toxicity. In these cases, it has been suggested that the toxic metabolite is produced more in the kidneys than in the liver.
The third phase follows at 3 to 5 days, and is marked by complications of massive hepatic necrosis
leading to fulminant hepatic failure with complications of coagulation
defects, hypoglycemia
, kidney failure, hepatic encephalopathy
, cerebral edema
, sepsis
, multiple organ failure, and death. If the third phase is survived, the hepatic necrosis runs its course, and liver and kidney function
typically return to normal in a few weeks. The severity of paracetamol toxicity varies depending on the dose and whether appropriate treatment is received.
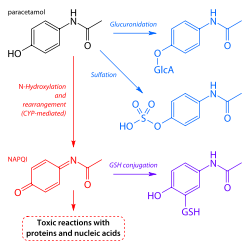 When taken in normal therapeutic doses, paracetamol has been shown to be safe. Following a therapeutic dose, it is mostly converted to nontoxic metabolites via Phase II metabolism by conjugation with sulfate
When taken in normal therapeutic doses, paracetamol has been shown to be safe. Following a therapeutic dose, it is mostly converted to nontoxic metabolites via Phase II metabolism by conjugation with sulfate
and glucuronide
, with a small portion being oxidized via the cytochrome P450
enzyme system. Cytochromes and 3A4
convert approximately 5% of paracetamol to a highly-reactive intermediary metabolite, N-acetyl-p-benzoquinoneimine
(NAPQI). Under normal conditions, NAPQI is detoxified by conjugation with glutathione
to form cysteine and mercapturic acid conjugates.
In cases of paracetamol overdose, the sulfate and glucuronide pathways become saturated, and more paracetamol is shunted to the cytochrome P450 system to produce NAPQI. As a result, hepatocellular supplies of glutathione become depleted, as the demand for glutathione is higher than its regeneration. NAPQI therefore remains in its toxic form in the liver and reacts with cellular membrane
molecules, resulting in widespread hepatocyte
damage and death, leading to acute hepatic necrosis. In animal studies, hepatic glutathione must be depleted to less than 70% of normal levels before hepatotoxicity occurs.
can induce
CYP2E1
, thus increasing the potential toxicity of paracetamol. Whether chronic alcoholism
should be considered a risk factor has been debated by some clinical toxicologists. For chronic alcohol users, acute alcohol ingestion at the time of a paracetamol overdose may have a protective effect. For non-chronic alcohol users, acute alcohol consumption had no protective effect.
Fasting
is a risk factor, possibly because of depletion of hepatic glutathione reserves. The concomitant use of the CYP2E1 inducer isoniazid
increases the risk of hepatotoxicity, though whether 2E1 induction is related to the hepatotoxicity in this case is unclear. Concomitant use of other drugs that induce CYP enzymes, such as antiepileptics including carbamazepine
, phenytoin
, and barbiturate
s, have also been reported as risk factors.
According to a preliminary study conducted by the University of Washington, mixing large amounts of both paracetamol and caffeine may cause liver damage. Researchers discovered that caffeine can triple the amount of NAPQI. This reaction can be caused by large doses of over-the-counter pain relief that combine caffeine and paracetamol. Dr. Sid Nelson, a professor of medicinal chemistry at the University of Washington, said, "Caffeine can interact with an enzyme that can form a toxic metabolite of paracetamol in such a way that it increases the formation of that toxic metabolite." However, the amount of caffeine that was shown to cause the effect in the study was an order of magnitude higher than typical doses experienced by coffee drinkers.
developed in 1975, called the Rumack-Matthew nomogram
, estimates the risk of toxicity based on the serum concentration of paracetamol at a given number of hours after ingestion. To determine the risk of potential hepatotoxicity, the paracetamol level is traced along the nomogram. Use of a timed serum paracetamol level plotted on the nomogram appears to be the best marker indicating the potential for liver injury. A paracetamol level drawn in the first four hours after ingestion may underestimate the amount in the system because paracetamol may still be in the process of being absorbed from the gastrointestinal tract
. Therefore a serum level taken before 4 hours is not recommended.
Clinical or biochemical evidence of liver toxicity may develop in one to four days, although, in severe cases, it may be evident in 12 hours. Right-upper-quadrant tenderness may be present and can aid in diagnosis. Laboratory studies may show evidence of hepatic necrosis with elevated AST
, ALT
, bilirubin
, and prolonged coagulation times, particularly an elevated prothrombin time
. After paracetamol overdose, when AST and ALT exceed 1000 IU/L, paracetamol-induced hepatotoxicity can be diagnosed. In some cases, the AST and ALT levels can exceed 10,000 IU/L.
and 500 mg paracetamol. Methionine is included in order to ensure that sufficient levels of glutathione
in the liver
are maintained in order to minimize the liver damage caused if a paracetamol overdose is taken.
Other attempts at minimizing paracetamol's adverse effects have been suggested including adding an emetic agent to tablets, reducing publicity about paracetamol, the inclusion of warnings on packs of paracetamol, and limiting the quantity of the drug sold. Few of these measures have been tried, as they either are not practical or have potential safety issues that make them unsuitable. Limiting the availability of paracetamol tablets has been attempted in some countries. In the UK, sales of over-the-counter paracetamol are restricted to packs of 32 tablets in pharmacies, and 16 tablets in non-pharmacy outlets. Pharmacists may provide up to 100 tablets for those with chronic conditions at the pharmacist's discretion. In Ireland, the limits are 24 and 12 tablets, respectively. It is unclear whether these interventions actually reduce poisoning deaths from paracetamol overdose.
One suggested method of prevention is to make paracetamol a prescription-only medicine, or to remove it entirely from the market. However, overdose is a relatively minor problem; for example, only 0.08% of the UK population present with paracetamol overdose each year. In contrast, paracetamol is a safe and effective medication that is taken without complications by millions of people. In addition, alternative pain relief
medications such as aspirin
are more toxic in overdose, whereas non-steroidal anti-inflammatory drugs are associated with more adverse effects following normal use.
Paracetamol ester prodrug with L-pyroglutamic acid (PCA), a biosynthetic precursors of glutathione, has been synthesized to reduce paracetamol hepatotoxicity and improve bioavailability. The toxicological studies of different paracetamol esters show that L-5-oxo-pyrrolidine-2-paracetamol carboxylate reduces toxicity after administration of an overdose of paracetamol to mice. The glutathione hepatic values in mice induced by intraperitoneal injection of the ester are superimposable with the GSH levels recorded in no-treated mice control group. The mice group treated with an equivalent dose of paracetamol showed a significative decrease of gluthathione of 35% (p<0.01 vs untreated control group). The oral LD50 was found to be greater than 2000 mg kg-1, whereas the intraperitoneal LD50 was 1900 mg kg-1. These results taken together with the good hydrolysis and bioavailability data show that this ester is a potential candidate as a prodrug of paracetamol.
, better known as stomach pumping, may be considered if the amount ingested is potentially life-threatening and the procedure can be performed within 60 minutes of ingestion. Activated charcoal is the most common gastrointestinal decontamination procedure as it adsorbs
paracetamol, reducing its gastrointestinal absorption. Administering activated charcoal also poses less risk of aspiration
than gastric lavage.
It appears that the most benefit from activated charcoal is gained if it is given within 30 minutes to two hours of ingestion. Administering activated charcoal later than 2 hours can be considered in patients that may have delayed gastric emptying due to co-ingested drugs or following ingestion of sustained- or delayed-release paracetamol preparations. Activated charcoal should also be administered if co-ingested drugs warrant decontamination. There was reluctance to give activated charcoal in paracetamol overdose, because of the concern that it may also absorb the oral antidote acetylcysteine. Studies have shown that 39% less acetylcysteine is absorbed into the body when they are administered together. There are conflicting recommendations regarding whether to change the dosing of oral acetylcysteine after the administration of activated charcoal, and even whether the dosing of acetylcysteine needs to be altered at all. Intravenous acetylcystine has no interaction with activated charcoal.
Inducing vomiting with syrup of ipecac
has no role in paracetamol overdose because the vomiting it induces delays the effective administration of activated charcoal and oral acetylcysteine. Liver injury is extremely rare after acute accidental ingestion in children under 6 years of age. Children with accidental exposures do not require gastrointestinal decontamination with either gastric lavage, activated charcoal, or syrup of ipecac.
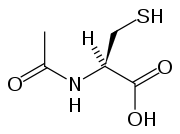 Acetylcysteine, also called N-acetylcysteine or NAC, works to reduce paracetamol toxicity by replenishing body stores of the antioxidant glutathione
Acetylcysteine, also called N-acetylcysteine or NAC, works to reduce paracetamol toxicity by replenishing body stores of the antioxidant glutathione
. Glutathione react with the toxic NAPQI metabolite so that it does not damage cells and can be safely excreted. Cysteamine
and methionine
have also been used to prevent hepatotoxicity, although studies show that both are associated with more adverse effects than acetylcysteine. Additionally, acetylcysteine has been shown to be a more effective antidote, particularly in patients presenting greater than 8 hours post-ingestion.
If the patient presents less than eight hours after paracetamol overdose, then acetylcysteine significantly reduces the risk of serious hepatotoxicity and guarantees survival. If acetylcysteine is started more than 8 hours after ingestion, there is a sharp decline in its effectiveness because the cascade of toxic events in the liver has already begun, and the risk of acute hepatic necrosis and death increases dramatically. Although acetylcysteine is most effective if given early, it still has beneficial effects if given as late as 48 hours after ingestion. In clinical practice, if the patient presents more than eight hours after the paracetamol overdose, then activated charcoal is not useful, and acetylcysteine is started immediately. In earlier presentations, charcoal can be given when the patient arrives and acetylcysteine is initiated while waiting for the paracetamol level results to return from the laboratory.
In United States practice, intravenous (IV) and oral administration are considered to be equally effective if given within 8 hours of ingestion. However, IV is the only recommended route in Australasian and British practice. Oral acetylcysteine is given as a 140 mg/kg loading dose
followed by 70 mg/kg every four hours for 17 more doses. Oral acetylcysteine may be poorly tolerated due to its unpleasant taste, odor, and its tendency to cause nausea and vomiting. If repeat doses of charcoal are indicated because of another ingested drug, then subsequent doses of charcoal and acetylcysteine should be staggered.
Intravenous acetylcysteine is given as a continuous infusion over 20 hours for a total dose 300 mg/kg. Recommended administration involves infusion of a 150 mg/kg loading dose over 15 to 60 minutes, followed by a 50 mg/kg infusion over four hours; the last 100 mg/kg are infused over the remaining 16 hours of the protocol. Intravenous acetylcysteine has the advantage of shortening hospital stay, increasing both doctor and patient convenience, and allowing administration of activated charcoal to reduce absorption of both the paracetamol and any co-ingested drugs without concerns about interference with oral acetylcysteine.
The most common adverse effect to acetylcysteine treatment is an anaphylactoid reaction, usually manifested by rash, wheeze, or mild hypotension
. Adverse reactions are more common in people treated with IV acetylcysteine, occurring in 4 to 23% of patients. Rarely, severe life-threatening reactions may occur in predisposed individuals, such as patients with asthma
. If a anaphylactoid reaction occurs the acetylcysteine is temporarily halted or slowed and antihistamine
s and other supportive care is administered.
. Liver transplants are performed in specialist centers. The most commonly used criteria for liver transplant was developed by physicians at King's College Hospital
in London. Patients are recommended for transplant if they have an arterial blood pH less than 7.3 after fluid resuscitation
or if a patient has Grade III or IV encephalopathy, a prothrombin time greater than 100 seconds, and a serum creatinine
greater than 300 mmol/L In a 24 hour period. Other forms of liver support have been used including partial liver transplants. These techniques have the advantage of supporting the patient while their own liver regenerates. Once liver function returns immunosuppressive drug
s are discontinued and they avoid taking immunosuppressive medication for the rest of their lives.
from paracetamol overdose increases two days after the ingestion, reaches a maximum on day four, and then gradually decreases. Acidemia is the most important single indicator of probable mortality and the need for transplantation. A mortality rate of 95% without transplant was reported in patients who had a documented pH
less than 7.30. Other indicators of poor prognosis include renal insufficiency, grade 3 or worse hepatic encephalopathy
, a markedly elevated prothrombin time, or an elevated blood lactic acid
level. One study has shown that a factor V
level less than 10% of normal indicated a poor prognosis (91% mortality), whereas a ratio of factor VIII
to factor V of less than 30 indicated a good prognosis (100% survival). Patients with a poor prognosis are usually identified for likely liver transplantation. Patients that do not die are expected to fully recover and have a normal life expectancy
and quality of life
.
and as prescription-only
medications. Because of its wide availability paired with comparably high toxicity, (compared to ibuprofen
and aspirin
) there is a much higher potential for overdose. Paracetamol toxicity is one of the most common causes of poisoning worldwide. In the United States, the United Kingdom, Australia, and New Zealand, paracetamol is the most common cause of drug overdoses. Additionally, in both the United States and the United Kingdom it is the most common cause of acute liver failure.
In England and Wales
an estimated 41,200 cases of paracetamol poisoning occurred in 1989 to 1990, with a mortality of 0.40%. It is estimated that 150 to 200 deaths and 15 to 20 liver transplants occur as a result of poisoning each year in England and Wales. Paracetamol overdose results in more calls to poison control center
s in the US than overdose of any other pharmacological substance, accounting for more than 100,000 calls, as well as 56,000 emergency room visits, 2,600 hospitalizations, and 458 deaths due to acute liver failure per year. A study of cases of acute liver failure between November 2000 and October 2004 by the Centers for Disease Control and Prevention
in the USA found that paracetamol was the cause of 41% of all cases in adults, and 25% of cases in children.
Analgesic
An analgesic is any member of the group of drugs used to relieve pain . The word analgesic derives from Greek an- and algos ....
drug paracetamol
Paracetamol
Paracetamol INN , or acetaminophen USAN , is a widely used over-the-counter analgesic and antipyretic . It is commonly used for the relief of headaches and other minor aches and pains and is a major ingredient in numerous cold and flu remedies...
(called acetaminophen in North America). Mainly causing liver injury, paracetamol toxicity is one of the most common causes of poisoning worldwide. In the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
and the United Kingdom
United Kingdom
The United Kingdom of Great Britain and Northern IrelandIn the United Kingdom and Dependencies, other languages have been officially recognised as legitimate autochthonous languages under the European Charter for Regional or Minority Languages...
it is the most common cause of acute liver failure.
Many individuals with paracetamol toxicity may have no symptoms at all in the first 24 hours following overdose. Others may initially have nonspecific complaints such as vague abdominal pain
Abdominal pain
Abdominal pain can be one of the symptoms associated with transient disorders or serious disease. Making a definitive diagnosis of the cause of abdominal pain can be difficult, because many diseases can result in this symptom. Abdominal pain is a common problem...
and nausea
Nausea
Nausea , is a sensation of unease and discomfort in the upper stomach with an involuntary urge to vomit. It often, but not always, precedes vomiting...
. With progressive disease, signs of liver failure may develop; these include low blood sugar
Hypoglycemia
Hypoglycemia or hypoglycæmia is the medical term for a state produced by a lower than normal level of blood glucose. The term literally means "under-sweet blood"...
, low blood pH
Metabolic acidosis
In medicine, metabolic acidosis is a condition that occurs when the body produces too much acid or when the kidneys are not removing enough acid from the body. If unchecked, metabolic acidosis leads to acidemia, i.e., blood pH is low due to increased production of hydrogen by the body or the...
, easy bleeding
Bleeding diathesis
In medicine , bleeding diathesis is an unusual susceptibility to bleeding mostly due to hypocoagulability, in turn caused by a coagulopathy . Several types are distinguished, ranging from mild to lethal...
, and hepatic encephalopathy
Hepatic encephalopathy
Hepatic encephalopathy is the occurrence of confusion, altered level of consciousness and coma as a result of liver failure. In the advanced stages it is called hepatic coma or coma hepaticum...
. Some will spontaneously resolve, although untreated cases may result in death.
Damage to the liver, or hepatotoxicity
Hepatotoxicity
Hepatotoxicity implies chemical-driven liver damage.The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses and sometimes even when introduced within therapeutic ranges, may injure...
, results not from paracetamol itself, but from one of its metabolite
Metabolite
Metabolites are the intermediates and products of metabolism. The term metabolite is usually restricted to small molecules. A primary metabolite is directly involved in normal growth, development, and reproduction. Alcohol is an example of a primary metabolite produced in large-scale by industrial...
s, N-acetyl-p-benzoquinoneimine
NAPQI
NAPQI is a toxic byproduct produced during the xenobiotic metabolism of the analgesic paracetamol...
(NAPQI). NAPQI depletes the liver's natural antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
and directly damages cells in the liver, leading to liver failure. Risk factors for toxicity include excessive chronic alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
intake, fasting
Fasting
Fasting is primarily the act of willingly abstaining from some or all food, drink, or both, for a period of time. An absolute fast is normally defined as abstinence from all food and liquid for a defined period, usually a single day , or several days. Other fasts may be only partially restrictive,...
or anorexia nervosa
Anorexia nervosa
Anorexia nervosa is an eating disorder characterized by refusal to maintain a healthy body weight and an obsessive fear of gaining weight. Although commonly called "anorexia", that term on its own denotes any symptomatic loss of appetite and is not strictly accurate...
, and the use of certain drugs such as isoniazid
Isoniazid
Isoniazid , also known as isonicotinylhydrazine , is an organic compound that is the first-line antituberculosis medication in prevention and treatment. It was first discovered in 1912, and later in 1951 it was found to be effective against tuberculosis by inhibiting its mycolic acid...
.
Treatment is aimed at removing the paracetamol from the body and replacing glutathione. Activated charcoal can be used to decrease absorption of paracetamol if the patient presents for treatment soon after the overdose; the antidote acetylcysteine acts as a precursor for glutathione, helping the body regenerate enough to prevent damage to the liver. A liver transplant is often required if damage to the liver becomes severe. Patients treated early have a good prognosis, whereas patients that develop major liver abnormalities typically have a poor outcome. Efforts to prevent paracetamol overdose include limiting individual sales of the drug and combining paracetamol with methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
, which is converted into glutathione in the liver.
Toxicity
The toxic dose of paracetamol is highly variable. In general the recommended maximum daily dose for healthy adults is 4 grams. Higher doses lead to increasing risk of toxicity. In adults, single doses above 10 grams or 200 mg/kg of bodyweight, whichever is lower, have a reasonable likelihood of causing toxicity. Toxicity can also occur when multiple smaller doses within 24 hours exceeds these levels. Following a normal dose of 1 gram of paracetamol four times a day for two weeks, patients can expect an increase in alanine transaminaseAlanine transaminase
Alanine transaminase or ALT is a transaminase enzyme . It is also called serum glutamic pyruvic transaminase or alanine aminotransferase ....
in their liver
Liver function tests
Liver function tests , are groups of clinical biochemistry laboratory blood assays designed to give information about the state of a patient's liver. The parameters measured include PT/INR, aPTT, albumin, billirubin and others...
to typically
Median
In probability theory and statistics, a median is described as the numerical value separating the higher half of a sample, a population, or a probability distribution, from the lower half. The median of a finite list of numbers can be found by arranging all the observations from lowest value to...
about three times the normal value. It is unlikely that this dose would lead to liver failure
Liver failure
Acute liver failure is the appearance of severe complications rapidly after the first signs of liver disease , and indicates that the liver has sustained severe damage . The complications are hepatic encephalopathy and impaired protein synthesis...
. Studies have shown significant hepatotoxicity is uncommon in patients who have taken greater than normal doses over 3 to 4 days. In adults, a dose of 6 grams a day over the preceding 48 hours could potentially lead to toxicity, while in children acute doses above 200 mg/kg could potentially cause toxicity. Acute paracetamol overdose in children rarely causes illness or death, and it is very uncommon for children to have levels that require treatment, with chronic larger-than-normal doses being the major cause of toxicity in children. Intravenous doses should be smaller than those taken orally, all other things being equal.
In rare individuals, paracetamol toxicity can result from normal use. This may be due to individual ("idiosyncratic") differences in the expression and activity of certain enzymes in one of the metabolic pathway
Metabolic pathway
In biochemistry, metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function...
s that handle paracetamol (see paracetamol's metabolism).
Signs and symptoms
The signs and symptoms of paracetamol toxicity occur in three phases. The first phase begins within hours of overdose, and consists of nauseaNausea
Nausea , is a sensation of unease and discomfort in the upper stomach with an involuntary urge to vomit. It often, but not always, precedes vomiting...
, vomiting
Vomiting
Vomiting is the forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose...
, pallor
Pallor
Pallor is a reduced amount of oxyhaemoglobin in skin or mucous membrane, a pale color which can be caused by illness, emotional shock or stress, stimulant use, lack of exposure to sunlight, anaemia or genetics....
, and sweating
Sweating
Perspiration is the production of a fluid consisting primarily of water as well as various dissolved solids , that is excreted by the sweat glands in the skin of mammals...
. However, patients often have no specific symptoms or only mild symptoms in the first 24 hours of poisoning. Rarely, after massive overdoses, patients may develop symptoms of metabolic acidosis
Metabolic acidosis
In medicine, metabolic acidosis is a condition that occurs when the body produces too much acid or when the kidneys are not removing enough acid from the body. If unchecked, metabolic acidosis leads to acidemia, i.e., blood pH is low due to increased production of hydrogen by the body or the...
and coma
Coma
In medicine, a coma is a state of unconsciousness, lasting more than 6 hours in which a person cannot be awakened, fails to respond normally to painful stimuli, light or sound, lacks a normal sleep-wake cycle and does not initiate voluntary actions. A person in a state of coma is described as...
early in the course of poisoning.
The second phase occurs between 24 and 72 hours following overdose and consists of signs of increasing liver damage. In general, damage occurs in hepatocyte
Hepatocyte
A hepatocyte is a cell of the main tissue of the liver. Hepatocytes make up 70-80% of the liver's cytoplasmic mass.These cells are involved in:* Protein synthesis* Protein storage* Transformation of carbohydrates...
s as they metabolize the paracetamol. The individual may experience right-upper-quadrant pain. The increasing liver damage also alters biochemical markers of liver function; International normalized ratio (INR) and the hepatic transaminase
Transaminase
In biochemistry, a transaminase or an aminotransferase is an enzyme that catalyzes a type of reaction between an amino acid and an α-keto acid. To be specific, this reaction involves removing the amino group from the amino acid, leaving behind an α-keto acid, and transferring it to the...
s alanine transaminase
Alanine transaminase
Alanine transaminase or ALT is a transaminase enzyme . It is also called serum glutamic pyruvic transaminase or alanine aminotransferase ....
and aspartate transaminase
Aspartate transaminase
Aspartate transaminase , also called aspartate aminotransferase or serum glutamic oxaloacetic transaminase , is a pyridoxal phosphate -dependent transaminase enzyme . AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in...
rise to abnormal levels. Acute kidney failure may also occur during this phase, typically caused by either hepatorenal syndrome
Hepatorenal syndrome
Hepatorenal syndrome is a life-threatening medical condition that consists of rapid deterioration in kidney function in individuals with cirrhosis or fulminant liver failure...
or multiple organ dysfunction syndrome
Multiple organ dysfunction syndrome
Multiple organ dysfunction syndrome ', previously known as multiple organ failure or multisystem organ failure , is altered organ function in an acutely ill patient requiring medical intervention to achieve homeostasis...
. In some cases, acute kidney failure may be the primary clinical manifestation of toxicity. In these cases, it has been suggested that the toxic metabolite is produced more in the kidneys than in the liver.
The third phase follows at 3 to 5 days, and is marked by complications of massive hepatic necrosis
Necrosis
Necrosis is the premature death of cells in living tissue. Necrosis is caused by factors external to the cell or tissue, such as infection, toxins, or trauma. This is in contrast to apoptosis, which is a naturally occurring cause of cellular death...
leading to fulminant hepatic failure with complications of coagulation
Coagulation
Coagulation is a complex process by which blood forms clots. It is an important part of hemostasis, the cessation of blood loss from a damaged vessel, wherein a damaged blood vessel wall is covered by a platelet and fibrin-containing clot to stop bleeding and begin repair of the damaged vessel...
defects, hypoglycemia
Hypoglycemia
Hypoglycemia or hypoglycæmia is the medical term for a state produced by a lower than normal level of blood glucose. The term literally means "under-sweet blood"...
, kidney failure, hepatic encephalopathy
Hepatic encephalopathy
Hepatic encephalopathy is the occurrence of confusion, altered level of consciousness and coma as a result of liver failure. In the advanced stages it is called hepatic coma or coma hepaticum...
, cerebral edema
Cerebral edema
Cerebral edema or cerebral œdema is an excess accumulation of water in the intracellular or extracellular spaces of the brain.-Vasogenic:Due to a breakdown of tight endothelial junctions which make up the blood-brain barrier...
, sepsis
Sepsis
Sepsis is a potentially deadly medical condition that is characterized by a whole-body inflammatory state and the presence of a known or suspected infection. The body may develop this inflammatory response by the immune system to microbes in the blood, urine, lungs, skin, or other tissues...
, multiple organ failure, and death. If the third phase is survived, the hepatic necrosis runs its course, and liver and kidney function
Renal physiology
Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including reabsorption of glucose, amino acids, and other small molecules; regulation of sodium, potassium, and other electrolytes; regulation of fluid balance and blood pressure;...
typically return to normal in a few weeks. The severity of paracetamol toxicity varies depending on the dose and whether appropriate treatment is received.
Pathophysiology

Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
and glucuronide
Glucuronide
A glucuronide, also known as glucuronoside, is any substance produced by linking glucuronic acid to another substance via a glycosidic bond...
, with a small portion being oxidized via the cytochrome P450
Cytochrome P450 oxidase
The cytochrome P450 superfamily is a large and diverse group of enzymes. The function of most CYP enzymes is to catalyze the oxidation of organic substances. The substrates of CYP enzymes include metabolic intermediates such as lipids and steroidal hormones, as well as xenobiotic substances...
enzyme system. Cytochromes and 3A4
CYP3A4
Cytochrome P450 3A4 , a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. CYP3A4 is involved in the oxidation of the largest range of substrates of all the CYPs. As a result, CYP3A4 is present in...
convert approximately 5% of paracetamol to a highly-reactive intermediary metabolite, N-acetyl-p-benzoquinoneimine
NAPQI
NAPQI is a toxic byproduct produced during the xenobiotic metabolism of the analgesic paracetamol...
(NAPQI). Under normal conditions, NAPQI is detoxified by conjugation with glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
to form cysteine and mercapturic acid conjugates.
In cases of paracetamol overdose, the sulfate and glucuronide pathways become saturated, and more paracetamol is shunted to the cytochrome P450 system to produce NAPQI. As a result, hepatocellular supplies of glutathione become depleted, as the demand for glutathione is higher than its regeneration. NAPQI therefore remains in its toxic form in the liver and reacts with cellular membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
molecules, resulting in widespread hepatocyte
Hepatocyte
A hepatocyte is a cell of the main tissue of the liver. Hepatocytes make up 70-80% of the liver's cytoplasmic mass.These cells are involved in:* Protein synthesis* Protein storage* Transformation of carbohydrates...
damage and death, leading to acute hepatic necrosis. In animal studies, hepatic glutathione must be depleted to less than 70% of normal levels before hepatotoxicity occurs.
Risk factors
A number of factors can potentially increase the risk of developing paracetamol toxicity. Chronic excessive alcohol consumptionAlcohol consumption and health
The long term effects of alcohol range from possible health benefits for low levels of alcohol consumption to severe detrimental effects in cases of chronic alcohol abuse...
can induce
Enzyme induction and inhibition
Enzyme induction is a process in which a molecule induces the expression of an enzyme.Enzyme inhibition can refer to* the inhibition of the expression of the enzyme by another molecule...
CYP2E1
CYP2E1
Cytochrome P450 2E1 , a member of the cytochrome P450 mixed-function oxidase system, is involved in the metabolism of xenobiotics in the body. In humans, the CYP2E1 enzyme is encoded by the CYP2E1 gene...
, thus increasing the potential toxicity of paracetamol. Whether chronic alcoholism
Alcoholism
Alcoholism is a broad term for problems with alcohol, and is generally used to mean compulsive and uncontrolled consumption of alcoholic beverages, usually to the detriment of the drinker's health, personal relationships, and social standing...
should be considered a risk factor has been debated by some clinical toxicologists. For chronic alcohol users, acute alcohol ingestion at the time of a paracetamol overdose may have a protective effect. For non-chronic alcohol users, acute alcohol consumption had no protective effect.
Fasting
Fasting
Fasting is primarily the act of willingly abstaining from some or all food, drink, or both, for a period of time. An absolute fast is normally defined as abstinence from all food and liquid for a defined period, usually a single day , or several days. Other fasts may be only partially restrictive,...
is a risk factor, possibly because of depletion of hepatic glutathione reserves. The concomitant use of the CYP2E1 inducer isoniazid
Isoniazid
Isoniazid , also known as isonicotinylhydrazine , is an organic compound that is the first-line antituberculosis medication in prevention and treatment. It was first discovered in 1912, and later in 1951 it was found to be effective against tuberculosis by inhibiting its mycolic acid...
increases the risk of hepatotoxicity, though whether 2E1 induction is related to the hepatotoxicity in this case is unclear. Concomitant use of other drugs that induce CYP enzymes, such as antiepileptics including carbamazepine
Carbamazepine
Carbamazepine is an anticonvulsant and mood-stabilizing drug used primarily in the treatment of epilepsy and bipolar disorder, as well as trigeminal neuralgia...
, phenytoin
Phenytoin
Phenytoin sodium is a commonly used antiepileptic. Phenytoin acts to suppress the abnormal brain activity seen in seizure by reducing electrical conductance among brain cells by stabilizing the inactive state of voltage-gated sodium channels...
, and barbiturate
Barbiturate
Barbiturates are drugs that act as central nervous system depressants, and can therefore produce a wide spectrum of effects, from mild sedation to total anesthesia. They are also effective as anxiolytics, as hypnotics, and as anticonvulsants...
s, have also been reported as risk factors.
According to a preliminary study conducted by the University of Washington, mixing large amounts of both paracetamol and caffeine may cause liver damage. Researchers discovered that caffeine can triple the amount of NAPQI. This reaction can be caused by large doses of over-the-counter pain relief that combine caffeine and paracetamol. Dr. Sid Nelson, a professor of medicinal chemistry at the University of Washington, said, "Caffeine can interact with an enzyme that can form a toxic metabolite of paracetamol in such a way that it increases the formation of that toxic metabolite." However, the amount of caffeine that was shown to cause the effect in the study was an order of magnitude higher than typical doses experienced by coffee drinkers.
Diagnosis
The most effective way to diagnose poisoning is by obtaining a blood paracetamol level. A drug nomogramNomogram
A nomogram, nomograph, or abac is a graphical calculating device developed by P.E. Elyasberg, a two-dimensional diagram designed to allow the approximate graphical computation of a function: it uses a coordinate system other than Cartesian coordinates...
developed in 1975, called the Rumack-Matthew nomogram
Rumack-Matthew nomogram
The Rumack-Matthew nomogram , also known as Rumack-Matthews nomogram or the Acetaminophen nomogram is an acetaminophen toxicity nomogram plotting serum concentration of acetaminophen against the time since the onset of ingestion in an attempt to prognosticate possible liver toxicity as well as...
, estimates the risk of toxicity based on the serum concentration of paracetamol at a given number of hours after ingestion. To determine the risk of potential hepatotoxicity, the paracetamol level is traced along the nomogram. Use of a timed serum paracetamol level plotted on the nomogram appears to be the best marker indicating the potential for liver injury. A paracetamol level drawn in the first four hours after ingestion may underestimate the amount in the system because paracetamol may still be in the process of being absorbed from the gastrointestinal tract
Gastrointestinal tract
The human gastrointestinal tract refers to the stomach and intestine, and sometimes to all the structures from the mouth to the anus. ....
. Therefore a serum level taken before 4 hours is not recommended.
Clinical or biochemical evidence of liver toxicity may develop in one to four days, although, in severe cases, it may be evident in 12 hours. Right-upper-quadrant tenderness may be present and can aid in diagnosis. Laboratory studies may show evidence of hepatic necrosis with elevated AST
Aspartate transaminase
Aspartate transaminase , also called aspartate aminotransferase or serum glutamic oxaloacetic transaminase , is a pyridoxal phosphate -dependent transaminase enzyme . AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in...
, ALT
Alanine transaminase
Alanine transaminase or ALT is a transaminase enzyme . It is also called serum glutamic pyruvic transaminase or alanine aminotransferase ....
, bilirubin
Bilirubin
Bilirubin is the yellow breakdown product of normal heme catabolism. Heme is found in hemoglobin, a principal component of red blood cells. Bilirubin is excreted in bile and urine, and elevated levels may indicate certain diseases...
, and prolonged coagulation times, particularly an elevated prothrombin time
Prothrombin time
The prothrombin time and its derived measures of prothrombin ratio and international normalized ratio are measures of the extrinsic pathway of coagulation. This test is also called "ProTime INR" and "INR PT". They are used to determine the clotting tendency of blood, in the measure of warfarin...
. After paracetamol overdose, when AST and ALT exceed 1000 IU/L, paracetamol-induced hepatotoxicity can be diagnosed. In some cases, the AST and ALT levels can exceed 10,000 IU/L.
Prevention
Besides preventing an overdose, one way to prevent liver damage may be the use of Paradote. Paradote is a combination tablet containing 100 mg methionineMethionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
and 500 mg paracetamol. Methionine is included in order to ensure that sufficient levels of glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
in the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
are maintained in order to minimize the liver damage caused if a paracetamol overdose is taken.
Other attempts at minimizing paracetamol's adverse effects have been suggested including adding an emetic agent to tablets, reducing publicity about paracetamol, the inclusion of warnings on packs of paracetamol, and limiting the quantity of the drug sold. Few of these measures have been tried, as they either are not practical or have potential safety issues that make them unsuitable. Limiting the availability of paracetamol tablets has been attempted in some countries. In the UK, sales of over-the-counter paracetamol are restricted to packs of 32 tablets in pharmacies, and 16 tablets in non-pharmacy outlets. Pharmacists may provide up to 100 tablets for those with chronic conditions at the pharmacist's discretion. In Ireland, the limits are 24 and 12 tablets, respectively. It is unclear whether these interventions actually reduce poisoning deaths from paracetamol overdose.
One suggested method of prevention is to make paracetamol a prescription-only medicine, or to remove it entirely from the market. However, overdose is a relatively minor problem; for example, only 0.08% of the UK population present with paracetamol overdose each year. In contrast, paracetamol is a safe and effective medication that is taken without complications by millions of people. In addition, alternative pain relief
Pain management
Pain management is a branch of medicine employing an interdisciplinary approach for easing the suffering and improving the quality of life of those living with pain. The typical pain management team includes medical practitioners, clinical psychologists, physiotherapists, occupational therapists,...
medications such as aspirin
Aspirin
Aspirin , also known as acetylsalicylic acid , is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. It was discovered by Arthur Eichengrun, a chemist with the German company Bayer...
are more toxic in overdose, whereas non-steroidal anti-inflammatory drugs are associated with more adverse effects following normal use.
Paracetamol ester prodrug with L-pyroglutamic acid (PCA), a biosynthetic precursors of glutathione, has been synthesized to reduce paracetamol hepatotoxicity and improve bioavailability. The toxicological studies of different paracetamol esters show that L-5-oxo-pyrrolidine-2-paracetamol carboxylate reduces toxicity after administration of an overdose of paracetamol to mice. The glutathione hepatic values in mice induced by intraperitoneal injection of the ester are superimposable with the GSH levels recorded in no-treated mice control group. The mice group treated with an equivalent dose of paracetamol showed a significative decrease of gluthathione of 35% (p<0.01 vs untreated control group). The oral LD50 was found to be greater than 2000 mg kg-1, whereas the intraperitoneal LD50 was 1900 mg kg-1. These results taken together with the good hydrolysis and bioavailability data show that this ester is a potential candidate as a prodrug of paracetamol.
Gastric decontamination
In adults, the initial treatment for paracetamol overdose is gastrointestinal decontamination. Paracetamol absorption from the gastrointestinal tract is complete within two hours under normal circumstances, so decontamination is most helpful if performed within this timeframe. Gastric lavageGastric lavage
Gastric lavage, also commonly called stomach pumping or Gastric irrigation, is the process of cleaning out the contents of the stomach. It has been used for over 200 years as a means of eliminating poisons from the stomach. Such devices are normally used on a person who has ingested a poison or...
, better known as stomach pumping, may be considered if the amount ingested is potentially life-threatening and the procedure can be performed within 60 minutes of ingestion. Activated charcoal is the most common gastrointestinal decontamination procedure as it adsorbs
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
paracetamol, reducing its gastrointestinal absorption. Administering activated charcoal also poses less risk of aspiration
Aspiration pneumonia
Aspiration pneumonia is bronchopneumonia that develops due to the entrance of foreign materials into the bronchial tree, usually oral or gastric contents...
than gastric lavage.
It appears that the most benefit from activated charcoal is gained if it is given within 30 minutes to two hours of ingestion. Administering activated charcoal later than 2 hours can be considered in patients that may have delayed gastric emptying due to co-ingested drugs or following ingestion of sustained- or delayed-release paracetamol preparations. Activated charcoal should also be administered if co-ingested drugs warrant decontamination. There was reluctance to give activated charcoal in paracetamol overdose, because of the concern that it may also absorb the oral antidote acetylcysteine. Studies have shown that 39% less acetylcysteine is absorbed into the body when they are administered together. There are conflicting recommendations regarding whether to change the dosing of oral acetylcysteine after the administration of activated charcoal, and even whether the dosing of acetylcysteine needs to be altered at all. Intravenous acetylcystine has no interaction with activated charcoal.
Inducing vomiting with syrup of ipecac
Syrup of ipecac
Syrup of ipecac , commonly referred to as ipecac, is derived from the dried rhizome and roots of the ipecacuanha plant, and is a well known emetic .-Preparation:...
has no role in paracetamol overdose because the vomiting it induces delays the effective administration of activated charcoal and oral acetylcysteine. Liver injury is extremely rare after acute accidental ingestion in children under 6 years of age. Children with accidental exposures do not require gastrointestinal decontamination with either gastric lavage, activated charcoal, or syrup of ipecac.
Acetylcysteine

Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
. Glutathione react with the toxic NAPQI metabolite so that it does not damage cells and can be safely excreted. Cysteamine
Cysteamine
Cysteamine is the chemical compound with the formula HSCH2CH2NH2. It is the simplest stable aminothiol and a degradation product of the amino acid cysteine. It is often used as the hydrochloride salt, HSCH2CH2NH3Cl....
and methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
have also been used to prevent hepatotoxicity, although studies show that both are associated with more adverse effects than acetylcysteine. Additionally, acetylcysteine has been shown to be a more effective antidote, particularly in patients presenting greater than 8 hours post-ingestion.
If the patient presents less than eight hours after paracetamol overdose, then acetylcysteine significantly reduces the risk of serious hepatotoxicity and guarantees survival. If acetylcysteine is started more than 8 hours after ingestion, there is a sharp decline in its effectiveness because the cascade of toxic events in the liver has already begun, and the risk of acute hepatic necrosis and death increases dramatically. Although acetylcysteine is most effective if given early, it still has beneficial effects if given as late as 48 hours after ingestion. In clinical practice, if the patient presents more than eight hours after the paracetamol overdose, then activated charcoal is not useful, and acetylcysteine is started immediately. In earlier presentations, charcoal can be given when the patient arrives and acetylcysteine is initiated while waiting for the paracetamol level results to return from the laboratory.
In United States practice, intravenous (IV) and oral administration are considered to be equally effective if given within 8 hours of ingestion. However, IV is the only recommended route in Australasian and British practice. Oral acetylcysteine is given as a 140 mg/kg loading dose
Loading dose
A loading dose is an initial higher dose of a drug that may be given at the beginning of a course of treatment before dropping down to a lower maintenance dose.A loading dose is most useful for drugs that are eliminated from the body relatively slowly...
followed by 70 mg/kg every four hours for 17 more doses. Oral acetylcysteine may be poorly tolerated due to its unpleasant taste, odor, and its tendency to cause nausea and vomiting. If repeat doses of charcoal are indicated because of another ingested drug, then subsequent doses of charcoal and acetylcysteine should be staggered.
Intravenous acetylcysteine is given as a continuous infusion over 20 hours for a total dose 300 mg/kg. Recommended administration involves infusion of a 150 mg/kg loading dose over 15 to 60 minutes, followed by a 50 mg/kg infusion over four hours; the last 100 mg/kg are infused over the remaining 16 hours of the protocol. Intravenous acetylcysteine has the advantage of shortening hospital stay, increasing both doctor and patient convenience, and allowing administration of activated charcoal to reduce absorption of both the paracetamol and any co-ingested drugs without concerns about interference with oral acetylcysteine.
The most common adverse effect to acetylcysteine treatment is an anaphylactoid reaction, usually manifested by rash, wheeze, or mild hypotension
Hypotension
In physiology and medicine, hypotension is abnormally low blood pressure, especially in the arteries of the systemic circulation. It is best understood as a physiologic state, rather than a disease. It is often associated with shock, though not necessarily indicative of it. Hypotension is the...
. Adverse reactions are more common in people treated with IV acetylcysteine, occurring in 4 to 23% of patients. Rarely, severe life-threatening reactions may occur in predisposed individuals, such as patients with asthma
Asthma
Asthma is the common chronic inflammatory disease of the airways characterized by variable and recurring symptoms, reversible airflow obstruction, and bronchospasm. Symptoms include wheezing, coughing, chest tightness, and shortness of breath...
. If a anaphylactoid reaction occurs the acetylcysteine is temporarily halted or slowed and antihistamine
Antihistamine
An H1 antagonist is a histamine antagonist of the H1 receptor that serves to reduce or eliminate effects mediated by histamine, an endogenous chemical mediator released during allergic reactions...
s and other supportive care is administered.
Liver transplant
In patients who develop fulminant hepatic failure or who are otherwise expected to die from liver failure, the mainstay of management is liver transplantationLiver transplantation
Liver transplantation or hepatic transplantation is the replacement of a diseased liver with a healthy liver allograft. The most commonly used technique is orthotopic transplantation, in which the native liver is removed and replaced by the donor organ in the same anatomic location as the original...
. Liver transplants are performed in specialist centers. The most commonly used criteria for liver transplant was developed by physicians at King's College Hospital
King's College Hospital
King's College Hospital is an acute care facility in the London Borough of Lambeth, referred to locally and by staff simply as "King's" or abbreviated internally to "KCH"...
in London. Patients are recommended for transplant if they have an arterial blood pH less than 7.3 after fluid resuscitation
Fluid replacement
Fluid replacement or fluid resuscitation is the medical practice of replenishing bodily fluid lost through sweating, bleeding, fluid shifts or other pathologic processes. Fluids can be replaced via oral administration , intravenous administration, rectally, or hypodermoclysis, the direct injection...
or if a patient has Grade III or IV encephalopathy, a prothrombin time greater than 100 seconds, and a serum creatinine
Creatinine
Creatinine is a break-down product of creatine phosphate in muscle, and is usually produced at a fairly constant rate by the body...
greater than 300 mmol/L In a 24 hour period. Other forms of liver support have been used including partial liver transplants. These techniques have the advantage of supporting the patient while their own liver regenerates. Once liver function returns immunosuppressive drug
Immunosuppressive drug
Immunosuppressive drugs or immunosuppressive agents are drugs that inhibit or prevent activity of the immune system. They are used in immunosuppressive therapy to:...
s are discontinued and they avoid taking immunosuppressive medication for the rest of their lives.
Prognosis
The mortality rateMortality rate
Mortality rate is a measure of the number of deaths in a population, scaled to the size of that population, per unit time...
from paracetamol overdose increases two days after the ingestion, reaches a maximum on day four, and then gradually decreases. Acidemia is the most important single indicator of probable mortality and the need for transplantation. A mortality rate of 95% without transplant was reported in patients who had a documented pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
less than 7.30. Other indicators of poor prognosis include renal insufficiency, grade 3 or worse hepatic encephalopathy
Hepatic encephalopathy
Hepatic encephalopathy is the occurrence of confusion, altered level of consciousness and coma as a result of liver failure. In the advanced stages it is called hepatic coma or coma hepaticum...
, a markedly elevated prothrombin time, or an elevated blood lactic acid
Lactic acid
Lactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3...
level. One study has shown that a factor V
Factor V
Factor V is a protein of the coagulation system, rarely referred to as proaccelerin or labile factor. In contrast to most other coagulation factors, it is not enzymatically active but functions as a cofactor...
level less than 10% of normal indicated a poor prognosis (91% mortality), whereas a ratio of factor VIII
Factor VIII
Factor VIII is an essential blood clotting factor also known as anti-hemophilic factor . In humans, Factor VIII is encoded by the F8 gene...
to factor V of less than 30 indicated a good prognosis (100% survival). Patients with a poor prognosis are usually identified for likely liver transplantation. Patients that do not die are expected to fully recover and have a normal life expectancy
Life expectancy
Life expectancy is the expected number of years of life remaining at a given age. It is denoted by ex, which means the average number of subsequent years of life for someone now aged x, according to a particular mortality experience...
and quality of life
Quality of life
The term quality of life is used to evaluate the general well-being of individuals and societies. The term is used in a wide range of contexts, including the fields of international development, healthcare, and politics. Quality of life should not be confused with the concept of standard of...
.
Epidemiology
Paracetamol is contained in many preparations, available as both over-the-counterOver-the-counter drug
Over-the-counter drugs are medicines that may be sold directly to a consumer without a prescription from a healthcare professional, as compared to prescription drugs, which may be sold only to consumers possessing a valid prescription...
and as prescription-only
Prescription drug
A prescription medication is a licensed medicine that is regulated by legislation to require a medical prescription before it can be obtained. The term is used to distinguish it from over-the-counter drugs which can be obtained without a prescription...
medications. Because of its wide availability paired with comparably high toxicity, (compared to ibuprofen
Ibuprofen
Ibuprofen is a nonsteroidal anti-inflammatory drug used for relief of symptoms of arthritis, fever, as an analgesic , especially where there is an inflammatory component, and dysmenorrhea....
and aspirin
Aspirin
Aspirin , also known as acetylsalicylic acid , is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. It was discovered by Arthur Eichengrun, a chemist with the German company Bayer...
) there is a much higher potential for overdose. Paracetamol toxicity is one of the most common causes of poisoning worldwide. In the United States, the United Kingdom, Australia, and New Zealand, paracetamol is the most common cause of drug overdoses. Additionally, in both the United States and the United Kingdom it is the most common cause of acute liver failure.
In England and Wales
England and Wales
England and Wales is a jurisdiction within the United Kingdom. It consists of England and Wales, two of the four countries of the United Kingdom...
an estimated 41,200 cases of paracetamol poisoning occurred in 1989 to 1990, with a mortality of 0.40%. It is estimated that 150 to 200 deaths and 15 to 20 liver transplants occur as a result of poisoning each year in England and Wales. Paracetamol overdose results in more calls to poison control center
Poison control center
A poison control center is a medical facility that is able to provide immediate, free, and expert treatment advice and assistance over the telephone in case of exposure to poisonous or hazardous substances...
s in the US than overdose of any other pharmacological substance, accounting for more than 100,000 calls, as well as 56,000 emergency room visits, 2,600 hospitalizations, and 458 deaths due to acute liver failure per year. A study of cases of acute liver failure between November 2000 and October 2004 by the Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
The Centers for Disease Control and Prevention are a United States federal agency under the Department of Health and Human Services headquartered in Druid Hills, unincorporated DeKalb County, Georgia, in Greater Atlanta...
in the USA found that paracetamol was the cause of 41% of all cases in adults, and 25% of cases in children.

