
Solid-oxide fuel cell
Encyclopedia
Fuel cell
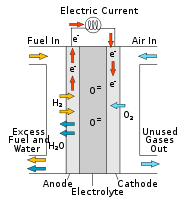
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
s are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic
Ceramic
A ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
, electrolyte. Advantages of this class of fuel cells include high efficiency, long-term stability, fuel flexibility, low emissions, and relatively low cost. The largest disadvantage is the high operating temperature
Operating temperature
An operating temperature is the temperature at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the device function and application context, and ranges from the minimum operating temperature to the...
which results in longer start-up times and mechanical and chemical compatibility issues.
Introduction
Solid oxide fuel cells are a class of fuel cell characterized by the use of a solid oxideOxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
material as the electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
. SOFCs use a solid oxide electrolyte to conduct negative oxygen ions from the cathode to the anode. The electrochemical oxidation of the oxygen ions with hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
or carbon monoxide thus occurs on the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
side. More recently, Proton Conducting SOFCs (PC-SOFC) are being developed which transport protons instead of oxygen ions through the electrolyte with the advantage of being able to be run at lower temperatures than traditional SOFCs.
They operate at very high temperatures, typically between 500 and 1,000 °C. At these temperatures, SOFCs do not require expensive platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
catalyst material, as is currently necessary for lower temperature fuel cells such as PEMFCs
Proton exchange membrane fuel cell
Proton exchange membrane fuel cells, also known as polymer electrolyte membrane fuel cells , are a type of fuel cell being developed for transport applications as well as for stationary fuel cell applications and portable fuel cell applications. Their distinguishing features include lower...
, and are not vulnerable to carbon monoxide catalyst poisoning. However, vulnerability to sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
poisoning has been widely observed and the sulfur must be removed before entering the cell through the use of adsorbent
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
beds or other means.
Solid oxide fuel cells have a wide variety of applications from use as auxiliary power units in vehicles to stationary power generation with outputs from 100 W to 2 MW. In 2009, Australian company, Ceramic Fuel Cells Ltd successfully achieved an efficiency of a SOFC device up to the previously theoretical mark of 60%. The higher operating temperature make SOFCs suitable candidates for application with heat engine
Heat engine
In thermodynamics, a heat engine is a system that performs the conversion of heat or thermal energy to mechanical work. It does this by bringing a working substance from a high temperature state to a lower temperature state. A heat "source" generates thermal energy that brings the working substance...
energy recovery devices or combined heat and power
Combined Heat and Power
Combined Heat and Power may refer to:* Cogeneration* Combined Heat and Power Solar...
, which further increases overall fuel efficiency.
Because of these high temperatures, light hydrocarbon fuels, such as methane, propane and butane can be internally reformed within the anode. SOFCs can also be fueled by externally reforming heavier hydrocarbons, such as gasoline, diesel, jet fuel (JP-8) or biofuels. Such reformates are mixtures of hydrogen, carbon monoxide, carbon dioxide, steam and methane, formed by reacting the hydrocarbon fuels with air or steam in a device upstream of the SOFC anode. SOFC power systems can increase efficiency by using the heat given off by the exothermic electrochemical oxidation within the fuel cell for endothermic steam reforming process.
Thermal expansion demands a uniform and well-regulated heating process at startup. SOFC stacks with planar geometry require on the order of an hour to be heated to light-off temperature. Micro-tubular fuel cell design geometries promise much faster start up times, typically on the order of minutes.
Unlike most other types of fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
s, SOFCs can have multiple geometries. The planar fuel cell design geometry is the typical sandwich type geometry employed by most types of fuel cells, where the electrolyte is sandwiched in between the electrodes. SOFCs can also be made in tubular geometries where either air or fuel is passed through the inside of the tube and the other gas is passed along the outside of the tube. The tubular design is advantageous because it is much easier to seal air from the fuel. The performance of the planar design is currently better than the performance of the tubular design however, because the planar design has a lower resistance comparatively. Other geometries of SOFCs include modified planar fuel cell designs (MPC or MPSOFC), where a wave-like structure replaces the traditional flat configuration of the planar cell. Such designs are highly promising, because they share the advantages of both planar cells (low resistance) and tubular cells.
Operation
Ceramic
A ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
s (hence the name). A single cell consisting of these four layers stacked together is typically only a few millimeters thick. Hundreds of these cells are then connected in series to form what most people refer to as an "SOFC stack". The ceramics used in SOFCs do not become electrically and ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
ically active until they reach very high temperature and as a consequence the stacks have to run at temperatures ranging from 500 to 1,000 °C. Reduction of oxygen into oxygen ions occurs at the cathode. These ions can then diffuse through the solid oxide electrolyte to the anode where they can electrochemically oxidize the fuel. In this reaction, a water byproduct is given off as well as two electrons. These electrons then flow through an external circuit where they can do work. The cycle then repeats as those electrons enter the cathode material again.
Balance of plant
Most of the downtime of a SOFC stems from the mechanical balance of plant, the air preheaterAir preheater
An air preheater is a general term to describe any device designed to heat air before another process with the primary objective of increasing the thermal efficiency of the process...
, prereformer, afterburner
AfterBurner
The AfterBurner is a lighting solution for the Game Boy Advance system that was created by Triton-Labs.Originally, portablemonopoly.net was a website created to petition Nintendo to put some kind of light in their Game Boy Advance system...
, water heat exchanger, anode tail gas oxidizer, and electrical balance of plant, power electronics
Power electronics
Power electronics is the application of solid-state electronics for the control and conversion of electric power.-Introduction:Power electronic converters can be found wherever there is a need to modify a form of electrical energy...
, hydrogen sulfide sensor
Hydrogen sulfide sensor
A hydrogen sulfide sensor or H2S sensor is a gas sensor for the measurement of hydrogen sulfide.-Principle:The H2S sensor is a metal oxide semiconductor sensor which operates by a reversible change in resistance caused by adsorption and desorption of hydrogen sulfide in a film with hydrogen...
and fans. Internal reforming leads to a large decrease in the balance of plant costs in designing a full system.
Anode
The ceramic anodeAnode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
layer must be very porous to allow the fuel to flow towards the electrolyte. Like the cathode, it must conduct electrons, with ionic conductivity a definite asset. The most common material used is a cermet
Cermet
A cermet is a composite material composed of ceramic and metallic materials. A cermet is ideally designed to have the optimal properties of both a ceramic, such as high temperature resistance and hardness, and those of a metal, such as the ability to undergo plastic deformation. The metal is used...
made up of nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
mixed with the ceramic material that is used for the electrolyte in that particular cell, typically YSZ (yttria stabilized zirconia), this YSZ part helps stop the grain growth of Nickel Ni. The anode is commonly the thickest and strongest layer in each individual cell, because it has the smallest polarization losses, and is often the layer that provides the mechanical support. Electrochemically
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
speaking, the anode’s job is to use the oxygen ions that diffuse through the electrolyte to oxidize the hydrogen fuel
Fuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
.
The oxidation reaction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
between the oxygen ions and the hydrogen produces heat as well as water and electricity.
If the fuel is a light hydrocarbon, for example methane, another function of the anode is to act as a catalyst for steam reforming the fuel into hydrogen. This provides another operational benefit to the fuel cell stack because the reforming reaction is endothermic, which cools the stack internally.
Electrolyte
The electrolyte is a dense layer of ceramic that conducts oxygen ions. Its electronic conductivity must be kept as low as possible to prevent losses from leakage currents. The high operating temperatures of SOFCs allow the kinetics of oxygen ion transport to be sufficient for good performance. However, as the operating temperature approaches the lower limit for SOFCs at around 600 °C, the electrolyte begins to have large ionic transport resistances and affect the performance. Popular electrolyte materials include yttria stabilized zirconia (YSZ) (often the 8% form Y8SZ), scandia stabilized zirconia (ScSZ) (usually 9 mol%Sc2O3 – 9ScSZ) and gadolinium doped ceria (GDC). The electrolyte material has crucial influence on the cell performances. Detrimental reactions between YSZ electrolytes and modern cathodes such as LSCF have been found, and can be prevented by thin (<100 nm) ceria diffusion barriers.If the conductivity for oxygen ions in SOFC can remain high even at lower temperature (current target in research ~500 °C), material choice for SOFC will broaden and many existing problems can potentially be solved. Certain processing technique such as thin film deposition can help solve this problem with existing material by
– reducing the traveling distance of oxygen ions and electrolyte resistance as resistance is inversely proportional to conductor length;
– producing grain structures that are less resistive such as columnar grain structure;
– controlling the micro-structural nano-crystalline fine grains to achieve "fine-tuning" of electrical properties;
– building composite with large interfacial areas as interfaces have shown to have extraordinary electrical properties.
Cathode
The cathodeCathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
, or air electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
, is a thin porous layer on the electrolyte where oxygen reduction takes place. The overall reaction is written in Kröger-Vink Notation
Kröger-Vink Notation
Kröger–Vink notation is set of conventions used to describe electric charge and lattice position for point defect species in crystals. It is primarily used for ionic crystals and is particularly useful for describing various defect reactions. It was proposed by F. A. Kröger and H. J. Vink.-General...
as follows:
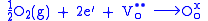
Cathode materials must be, at minimum, electronically conductive. Currently, lanthanum strontium manganite
Lanthanum strontium manganite
Lanthanum strontium manganite is an oxide ceramic material with the general formula La1-xSrxMnO3, where x describes the doping level and is usually in the range of 10-20%....
(LSM) is the cathode material of choice for commercial use because of its compatibility with doped zirconia electrolytes. Mechanically, it has similar coefficient of thermal expansion to YSZ and thus limits stresses built up because of CTE mismatch. Also, LSM has low levels of chemical reactivity with YSZ which extends the lifetime of the material. Unfortunately, LSM is a poor ionic conductor, and so the electrochemically active reaction is limited to the triple phase boundary (TPB) where the electrolyte, air and electrode meet. LSM works well as a cathode at high temperatures, but its performance quickly falls as the operating temperature is lowered below 800 °C. In order to increase the reaction zone beyond the TPB, a potential cathode material must be able to conduct both electrons and oxygen ions. Composite cathodes consisting of LSM YSZ have been used to increase this triple phase boundary length. Mixed ionic/electronic conducting (MIEC) ceramics, such as the perovskite LSCF, are also being researched for use in intermediate temperature SOFCs as they are more active and can makeup for the increase in the activation energy of reaction.
Interconnect
The interconnect can be either a metallic or ceramic layer that sits between each individual cell. Its purpose is to connect each cell in series, so that the electricity each cell generates can be combined. Because the interconnect is exposed to both the oxidizing and reducing side of the cell at high temperatures, it must be extremely stable. For this reason, ceramics have been more successful in the long term than metals as interconnect materials. However, these ceramic interconnect materials are very expensive as compared to metals. Nickel- and steel-based alloys are becoming more promising as lower temperature (600–800 °C) SOFCs are developed. The material of choice for an interconnect in contact with Y8SZ is a metallic 95Cr-5Fe alloy. Ceramic-metal composites called 'cermet' are also under consideration, as they have demonstrated thermal stability at high temperatures and excellent electrical conductivity.Polarizations
Polarizations, or overpotentials, are losses in voltage due to imperfections in materials, microstructure, and design of the fuel cell. Polarizations result from ohmic resistance of oxygen ions conducting through the electrolyte (iRΩ), electrochemical activation barriers at the anode and cathode, and finally concentration polarizations due to inability of gases to diffuse at high rates through the porous anode and cathode (shown as ηA for the anode and ηC for cathode). The cell voltage can be calculated using the following equation: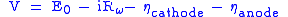
where
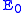 is the Nernst potential of the reactants and R represents the Thévenin equivalent
is the Nernst potential of the reactants and R represents the Thévenin equivalentThévenin's theorem
In circuit theory, Thévenin's theorem for linear electrical networks states that any combination of voltage sources, current sources, and resistors with two terminals is electrically equivalent to a single voltage source V and a single series resistor R. For single frequency AC systems the theorem...
resistance value of the electrically conducting portions of the cell.
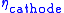 and
and 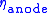 account for the remaining difference between the actual cell voltage and the Nernst potential. In SOFCs, it is often important to focus on the ohmic and concentration polarizations since high operating temperatures experience little activation polarization. However, as the lower limit of SOFC operating temperature is approached (~600 °C), these polarizations do become important.
account for the remaining difference between the actual cell voltage and the Nernst potential. In SOFCs, it is often important to focus on the ohmic and concentration polarizations since high operating temperatures experience little activation polarization. However, as the lower limit of SOFC operating temperature is approached (~600 °C), these polarizations do become important.Above mentioned equation is used for determining the SOFC voltage (in fact for fuel cell voltage in general). This approach results in good agreement with particular experimental data (for which
adequate factors were obtained) and poor agreement for other than original experimental working parameters. Moreover, most of the equations used require the addition of numerous factors which are difficult or impossible to determine. It makes very difficult any optimizing process of the SOFC working parameters as well as design architecture configuration selection. Because of those circumstances a few other equations were proposed:
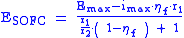
where:
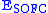 – cell voltage,
– cell voltage,  – maximum voltage given by the Nernst equation,
– maximum voltage given by the Nernst equation,  – maximum current density (for given fuel flow),
– maximum current density (for given fuel flow), 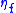 – fuel utilization factor,
– fuel utilization factor, 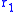 – ionic specific resistance of the electrolyte, and
– ionic specific resistance of the electrolyte, and 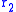 – electric specific resistance of the electrolyte.
– electric specific resistance of the electrolyte.There are many parameters which impact cell working conditions, e.g. electrolyte material, electrolyte thickness, cell temperature, inlet and outlet gas compositions at anode and cathode, and electrode porosity, just to name some. The flow in these systems is often calculated using the Navier-stokes equation.
Ohmic polarization
Ohmic losses in an SOFC result from ionic conductivity through the electrolyte. This is inherently a materials property of the crystal structure and atoms involved. However, to maximize the ionic conductivity, several methods can be done. Firstly, operating at higher temperatures can significantly decrease these ohmic losses. Substitutional doping methods to further refine the crystal structure and control defect concentrations can also play a significant role in increasing the conductivity. Another way to decrease ohmic resistance is to decrease the thickness of the electrolyte layer.Ionic conductivity
An ionic specific resistance of the electrolyte as a function of temperature can be described by the following relationship: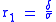
where:
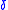 – electrolyte thickness, and
– electrolyte thickness, and  – ionic conductivity.
– ionic conductivity.The ionic conductivity of the solid oxide is defined as follows:
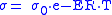
where:
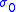 and
and  – factors depended on electrolyte materials,
– factors depended on electrolyte materials,  – electrolyte temperature, and
– electrolyte temperature, and  – ideal gas constant.
– ideal gas constant.Concentration polarization
The concentration polarization is the result of practical limitations on mass transport within the cell, and represents the voltage loss due to spatial variations in reactant concentration at the chemically active sites. This situation can be caused when the reactants are consumed by the electrochemical reaction faster than they can diffuse into the porous electrode, and can also be caused by variation in bulk flow composition. The latter is due to the fact that the consumption of reacting species in the reactant flows causes a drop in reactant concentration as it travels along the cell, which causes a drop in the local potential near the tail end of the cell.The concentration polarization occurs in both the anode and cathode. The anode can be particularly problematic, as the oxidation of the hydrogen produces steam, which further dilutes the fuel stream as it travels along the length of the cell. This polarization can be mitigated by reducing the reactant utilization fraction or increasing the electrode porosity, but these approaches each have significant design trade-offs.
Activation polarization
The activation polarization is the result of the kinetics involved with the electrochemical reactions. Each reaction has a certain activation barrier that must be overcome in order to proceed and this barrier leads to the polarization. The activation barrier is the result of many complex electrochemical reaction steps where typically the rate limiting step is responsible for the polarization. The polarization equation shown below is found by solving the Butler–Volmer equation in the high current density regime (where the cell typically operates), and can be used to estimate the activation polarization: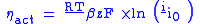
where:
 = gas constant
= gas constant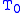 = operating temperature
= operating temperature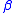 = electron transfer coefficient
= electron transfer coefficient = electrons associated with the electrochemical reaction
= electrons associated with the electrochemical reaction = Faraday's constant
= Faraday's constant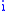 = operating current
= operating current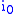 = exchange current density.
= exchange current density.
The polarization can be modified by microstructural optimization. The Triple Phase Boundary (TPB) length, which is the length where porous, ionic and electronically conducting pathways all meet, directly relates to the electrochemically active length in the cell. The larger the length, the more reactions can occur and thus the less the activation polarization. Optimization of TPB length can be done by processing conditions to affect microstructure or by materials selection to use a mixed ionic/electronic conductor to further increase TPB length.
Target
DOEUnited States Department of Energy
The United States Department of Energy is a Cabinet-level department of the United States government concerned with the United States' policies regarding energy and safety in handling nuclear material...
target requirements are 40,000 hours of service for stationary fuel cell applications and greater than 5,000 hours for transportation systems (fuel cell vehicle
Fuel cell vehicle
A Fuel cell vehicle or Fuel Cell Electric Vehicle is a type of hydrogen vehicle which uses a fuel cell to produce electricity, powering its on-board electric motor...
s) at a factory cost of $400/kW for a 10 kW coal
Coal
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
-based system without additional requirements. Lifetime effects (phase stability, thermal expansion compatibility, element migration, conductivity and aging) must be addressed. The Solid State Energy Conversion Alliance 2008 (interim) target for overall degradation per 1,000 hours is 4.0%.
Research
Research is going now in the direction of lower-temperature SOFC (600 °C) in order to decrease the materials cost, which will enable the use of metallic materials with better mechanical properties and thermal conductivityThermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
.
Research is currently underway to improve the fuel flexibility of SOFCs. While stable operation has been achieved on a variety of hydrocarbon fuels, these cells typically rely on external fuel processing. For the case of natural gas
Natural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
, the fuel is either externally or internally reformed and the sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
compounds are removed. These processes add to the cost and complexity of SOFC systems. Work is underway at a number of institutions to improve the stability of anode materials for hydrocarbon oxidation and, therefore, relax the requirements for fuel processing and decrease SOFC balance of plant costs.
Research is also going on in reducing start-up time to be able to implement SOFCs in mobile applications. Due to their fuel flexibility they may run on partially reformed diesel, and this makes SOFCs interesting as auxiliary power units (APU) in refrigerated trucks.
Specifically, Delphi Automotive Systems are developing an SOFC that will power auxiliary units in automobiles and tractor-trailers, while BMW
BMW
Bayerische Motoren Werke AG is a German automobile, motorcycle and engine manufacturing company founded in 1916. It also owns and produces the Mini marque, and is the parent company of Rolls-Royce Motor Cars. BMW produces motorcycles under BMW Motorrad and Husqvarna brands...
has recently stopped a similar project. A high-temperature SOFC will generate all of the needed electricity to allow the engine to be smaller and more efficient. The SOFC would run on the same gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
or diesel as the engine and would keep the air conditioning unit and other necessary electrical systems running while the engine shuts off when not needed (e.g., at a stop light or truck stop).
Rolls-Royce
Rolls-Royce plc
Rolls-Royce Group plc is a global power systems company headquartered in the City of Westminster, London, United Kingdom. It is the world’s second-largest maker of aircraft engines , and also has major businesses in the marine propulsion and energy sectors. Through its defence-related activities...
is developing solid-oxide fuel cells produced by screen printing onto inexpensive ceramic materials. Rolls-Royce Fuel Cell Systems Ltd is developing a SOFC gas turbine hybrid system fueled by natural gas for power generation applications on the order of a megawatt (e.g. Futuregen
FutureGen
FutureGen is a US government project announced by President George W. Bush in 2003; its initial plan involved the construction of a near zero-emissions coal-fueled power plant to produce hydrogen and electricity while using carbon capture and storage....
).
Ceres Power
Ceres Power
Ceres Power is a UK-based company that is developing a fuel-cell boiler for the UK domestic CHP market.The boiler produces both heat and electricity. The design is small enough to be wall-mounted and is therefore compatible with the layout of most central heating systems in the UK.At the heart of...
Ltd. has developed a low cost and low temperature (500–600 degrees) SOFC stack using cerium gadolinium oxide (CGO) in place of current industry standard ceramic, yttria stabilized zirconia (YSZ), which allows the use of stainless steel
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
to support the ceramic.
Solid Cell Inc. has developed a unique, low cost cell architecture that combines properties of planar and tubular designs, along with a Cr-free cermet
Cermet
A cermet is a composite material composed of ceramic and metallic materials. A cermet is ideally designed to have the optimal properties of both a ceramic, such as high temperature resistance and hardness, and those of a metal, such as the ability to undergo plastic deformation. The metal is used...
interconnect.
The high temperature electrochemistry center (HITEC) at the University of Florida, Gainesville is focused on studying ionic transport, electrocatalytic phenomena and microstructural characterization of ion conducting materials.
SiEnergy Systems, a Harvard spin-off company, has demonstrated the first macro-scale thin-film solid-oxide fuel cell that can operate at 500 degrees.
SOEC
A solid oxide electrolyser cellSolid oxide electrolyser cell
A solid oxide electrolyzer cell is a solid oxide fuel cell that is run in regenerative mode to achieve the electrolysis of water and which uses a solid oxide, or ceramic, electrolyte to produce oxygen and hydrogen gas.-Principle:...
(SOEC) is a solid oxide fuel cell set in regenerative mode for the electrolysis of water
Electrolysis of water
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water.-Principle:...
with a solid oxide, or ceramic
Ceramic
A ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
, electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
to produce oxygen and hydrogen gas.
ITSOFC
SOFCs that operate in an intermediate temperature (IT) range, meaning between 600 and 800 °C, are named ITSOFCs. Because of the high degradation rates and materials costs incurred at temperatures in excess of 900 °C, it is economically more favorable to operate SOFCs at lower temperatures. The push for high performance ITSOFCs is currently the topic of much research and development. One area of focus is the cathode material. It is thought that the oxygen reduction reaction is responsible for much of the loss in performance so the catalytic activity of the cathode is being studied and enhanced through various techniques, including catalyst impregnation.SOFC-GT
An SOFC-GT system is one which comprises a solid oxide fuel cell combined with a gas turbine. Such systems have been evaluated by Siemens Westinghouse and Rolls-RoyceRolls-Royce plc
Rolls-Royce Group plc is a global power systems company headquartered in the City of Westminster, London, United Kingdom. It is the world’s second-largest maker of aircraft engines , and also has major businesses in the marine propulsion and energy sectors. Through its defence-related activities...
as a means to achieve higher operating efficiencies by running the SOFC under pressure. SOFC-GT systems typically include anodic and/or cathodic atmosphere recirculation, thus increasing efficiency
Efficiency
Efficiency in general describes the extent to which time or effort is well used for the intended task or purpose. It is often used with the specific purpose of relaying the capability of a specific application of effort to produce a specific outcome effectively with a minimum amount or quantity of...
.
Theoretically, the combination of the SOFC and gas turbine can give result in high overall (electrical and thermal) efficiency. Further combination of the SOFC-GT in a combined heat and power configuration (via HVAC) also has the potential to yield even higher thermal efficiencies in some cases.
See also
- Auxiliary power unitAuxiliary power unitAn auxiliary power unit is a device on a vehicle that provides energy for functions other than propulsion. They are commonly found on large aircraft, as well as some large land vehicles.-Function:...
- Ceramic Fuel CellsCeramic Fuel CellsCeramic Fuel Cells Ltd is an Australian fuel cell technology company, based in Melbourne. The company produces the "BlueGen" gas-to-electricity generators. CFCL's develops solid oxide fuel cell technology to provide reliable, energy efficient, high quality, and low-emission electricity from...
Ltd – Australian company producing solid oxide fuel cells - Glossary of fuel cell termsGlossary of fuel cell termsThe Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few. –...
- Hydrogen technologiesHydrogen technologiesHydrogen technologies are technologies that relate to the production and use of hydrogen. Hydrogen technologies are applicable for many uses....
- Micro combined heat and power
External links
- US Department of Energy page on SOFCs
- An article in Encyclopedia at YCES
- Illinois Institute of Technology page on SOFCs
- Assessment of Solid Oxide Fuel Cells in Building Applications Phase 1: Modeling and Preliminary Analyses
- CSA Overview of SOFCs
- SOFC glass-ceramic sealing
- Refractory Specialties Inc.
- Materials & Systems Research, Inc.'s (MSRI)
- Solid Oxide Fuel Cells Canada (SOFCC) Strategic Research Network
- SOFC Dynamics and Control Research

