
Theta solvent
Encyclopedia
In a polymer
solution
, a theta solvent (or θ solvent) is a solvent
in which polymer coils act like ideal chain
s, assuming exactly their random walk
coil dimensions. Thermodynamically, the excess chemical potential
of mixing between a polymer and a theta solvent is zero.
assumed by a polymer chain in dilute solution can be modeled as a random walk of monomer
subunits using a freely jointed chain model. However, this model does not account for steric effects
. Real polymer coils are more closely represented by a self-avoiding walk
because conformations in which different chain segments occupy the same space are not physically possible. This excluded volume
effect causes the polymer to expand.
Chain conformation is also affected by solvent quality. The intermolecular interactions between polymer chain segments and coordinated solvent molecules have an associated energy of interaction which can be positive or negative. For a good solvent, interactions between polymer segments and solvent molecules are energetically favorable, and will cause polymer coils to expand. For a poor solvent, polymer-polymer self-interactions are preferred, and the polymer coils will contract. The quality of the solvent depends on both the chemical compositions of the polymer and solvent molecules and the solution temperature.
If a solvent is precisely poor enough to cancel the effects of excluded volume expansion, the theta (θ) condition is satisfied. For a given polymer-solvent pair, the theta condition is satisfied at a certain temperature, called the theta (θ) temperature or theta point. A solvent at this temperature is called a theta solvent.
In general, measurements of the properties of polymer solutions depend on the solvent. However, when a theta solvent is used, the measured characteristics are independent of the solvent. They depend only on short-range properties of the polymer such as the bond length, bond angles, and sterically favorable rotations. The polymer chain will behave exactly as predicted by the random walk or ideal chain
model. This makes experimental determination of important quantities such as the root mean square
end-to-end distance or the radius of gyration
much simpler.
Additionally, the theta condition is also satisfied in the bulk amorphous polymer phase
. Thus, the conformations adopted by polymers dissolved in theta solvents are identical to those adopted in bulk polymer.
of mixing is zero making the solution ideal
.
The chemical potential cannot be measured by any direct means, but it can be correlated to the osmotic pressure
,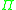 , of the solution and the partial specific volume
, of the solution and the partial specific volume
,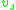 , of the solvent:
, of the solvent:
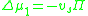
The concentration dependence of the osmotic pressure can be written as a virial expansion
,
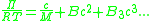
where M is the molecular weight of the polymer, R is the gas constant
, T is the absolute temperature, and B is the second virial coefficient
. Because the change in the chemical potential upon mixing is made up of an ideal term and an excess term,

the second virial coefficient, B, is proportional to the excess chemical potential of mixing:
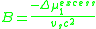
This parameter reflects the energy of binary interactions between polymer chain segments and solvent molecules. When B > 0, the solvent is “good,” and when B < 0, the solvent is “poor.” For a theta solvent, the second virial coefficient is equal to zero because the excess chemical potential is zero by definition. A solvent at its theta temperature is in this respect analogous to a real gas
at its Boyle temperature.
This relationship with osmotic pressure provides one means by which the theta condition (ie. the theta temperature for a particular solvent) can be determined. Similar relationships exist for other experimental techniques
including light scattering
, intrinsic viscosity
measurement, sedimentation equilibrium
, and cloud point titration.
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
, a theta solvent (or θ solvent) is a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
in which polymer coils act like ideal chain
Ideal chain
An ideal chain is the simplest model to describe a polymer. It only assumes a polymer as a random walk and neglects any kind of interactions among monomers...
s, assuming exactly their random walk
Random walk
A random walk, sometimes denoted RW, is a mathematical formalisation of a trajectory that consists of taking successive random steps. For example, the path traced by a molecule as it travels in a liquid or a gas, the search path of a foraging animal, the price of a fluctuating stock and the...
coil dimensions. Thermodynamically, the excess chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
of mixing between a polymer and a theta solvent is zero.
Physical Interpretation
The conformationChemical structure
A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
assumed by a polymer chain in dilute solution can be modeled as a random walk of monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
subunits using a freely jointed chain model. However, this model does not account for steric effects
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
. Real polymer coils are more closely represented by a self-avoiding walk
Self-avoiding walk
In mathematics, a self-avoiding walk is a sequence of moves on a lattice that does not visit the same point more than once. A self-avoiding polygon is a closed self-avoiding walk on a lattice...
because conformations in which different chain segments occupy the same space are not physically possible. This excluded volume
Excluded volume
The concept of excluded volume was introduced by Werner Kuhn in 1934 and applied to polymer molecules shortly thereafter by Paul Flory.- In liquid state theory :...
effect causes the polymer to expand.
Chain conformation is also affected by solvent quality. The intermolecular interactions between polymer chain segments and coordinated solvent molecules have an associated energy of interaction which can be positive or negative. For a good solvent, interactions between polymer segments and solvent molecules are energetically favorable, and will cause polymer coils to expand. For a poor solvent, polymer-polymer self-interactions are preferred, and the polymer coils will contract. The quality of the solvent depends on both the chemical compositions of the polymer and solvent molecules and the solution temperature.
If a solvent is precisely poor enough to cancel the effects of excluded volume expansion, the theta (θ) condition is satisfied. For a given polymer-solvent pair, the theta condition is satisfied at a certain temperature, called the theta (θ) temperature or theta point. A solvent at this temperature is called a theta solvent.
In general, measurements of the properties of polymer solutions depend on the solvent. However, when a theta solvent is used, the measured characteristics are independent of the solvent. They depend only on short-range properties of the polymer such as the bond length, bond angles, and sterically favorable rotations. The polymer chain will behave exactly as predicted by the random walk or ideal chain
Ideal chain
An ideal chain is the simplest model to describe a polymer. It only assumes a polymer as a random walk and neglects any kind of interactions among monomers...
model. This makes experimental determination of important quantities such as the root mean square
Root mean square
In mathematics, the root mean square , also known as the quadratic mean, is a statistical measure of the magnitude of a varying quantity. It is especially useful when variates are positive and negative, e.g., sinusoids...
end-to-end distance or the radius of gyration
Radius of gyration
Radius of gyration or gyradius is the name of several related measures of the size of an object, a surface, or an ensemble of points. It is calculated as the root mean square distance of the objects' parts from either its center of gravity or an axis....
much simpler.
Additionally, the theta condition is also satisfied in the bulk amorphous polymer phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
. Thus, the conformations adopted by polymers dissolved in theta solvents are identical to those adopted in bulk polymer.
Thermodynamic Definition
Thermodynamically, the excess chemical potential of mixing between a polymer and theta solvent is zero. Equivalently the enthalpyEnthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
of mixing is zero making the solution ideal
Ideal solution
In chemistry, an ideal solution or ideal mixture is a solution with thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of solution is zero as is the volume change on mixing; the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the...
.
The chemical potential cannot be measured by any direct means, but it can be correlated to the osmotic pressure
Osmotic pressure
Osmotic pressure is the pressure which needs to be applied to a solution to prevent the inward flow of water across a semipermeable membrane....
,
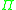 , of the solution and the partial specific volume
, of the solution and the partial specific volumePartial specific volume
The partial specific volume or PSV is a measure of the density of the particle using its calculated volume and mass information. The PSV is usually written in terms of milliLiters per gram .Partial specific volume is an inverse of density....
,
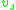 , of the solvent:
, of the solvent: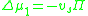
The concentration dependence of the osmotic pressure can be written as a virial expansion
Virial expansion
The classical virial expansion expresses the pressure of a many-particle system in equilibrium as a power series in the density.The virial expansion was introduced in 1901 by Heike Kamerlingh Onnesas a generalization of the ideal gas law...
,
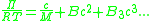
where M is the molecular weight of the polymer, R is the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
, T is the absolute temperature, and B is the second virial coefficient
Virial coefficient
Virial coefficients B_i appear as coefficients in the virial expansion of the pressure of a many-particle system in powers of the density, providing systematic corrections to the ideal gas law...
. Because the change in the chemical potential upon mixing is made up of an ideal term and an excess term,

the second virial coefficient, B, is proportional to the excess chemical potential of mixing:
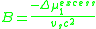
This parameter reflects the energy of binary interactions between polymer chain segments and solvent molecules. When B > 0, the solvent is “good,” and when B < 0, the solvent is “poor.” For a theta solvent, the second virial coefficient is equal to zero because the excess chemical potential is zero by definition. A solvent at its theta temperature is in this respect analogous to a real gas
Real gas
Real gases – as opposed to a perfect or ideal gas – exhibit properties that cannot be explained entirely using the ideal gas law. To understand the behaviour of real gases, the following must be taken into account:* compressibility effects;...
at its Boyle temperature.
This relationship with osmotic pressure provides one means by which the theta condition (ie. the theta temperature for a particular solvent) can be determined. Similar relationships exist for other experimental techniques
Experimental techniques
Experimental research designs are used for the controlled testing of causal processes.The general procedure is one or more independent variables are manipulated to determine their effect on a dependent variable...
including light scattering
Light scattering
Light scattering is a form of scattering in which light is the form of propagating energy which is scattered. Light scattering can be thought of as the deflection of a ray from a straight path, for example by irregularities in the propagation medium, particles, or in the interface between two media...
, intrinsic viscosity
Intrinsic viscosity
Intrinsic viscosity \left[ \eta \right] is a measure of a solute's contribution to the viscosity \eta of a solution. Intrinsic viscosity is frequently referred to as "Inherent Viscosity" in macromolecular literature...
measurement, sedimentation equilibrium
Sedimentation equilibrium
Sedimentation equilibrium in a solution or suspension of different particles, such as molecules, exists when the rate of transport of each material in any one direction due to sedimentation equals the rate of transport in the opposite direction due to diffusion...
, and cloud point titration.

