
Tunnel ionization
Encyclopedia
Tunnel ionization is a process in which electron
s in an atom
(or a molecule
) pass through the potential barrier and escape from the atom (or molecule). In an intense electric field
, the potential barrier of an atom (molecule) is distorted drastically. Therefore, the length of the barrier that electrons have to pass decreases and electrons can escape from the atom (molecule) easily.
As an electric field of light is an alternating electric field, the direction of the electric field reverses after the half period of the field. Because electrons have a charge
, electrons escaping by tunnel ionization come and go to the atom (molecule) in every half period.
In this process, some electrons recombine with the nucleus
(nuclei). Because the electrons have gained a large quantity of kinetic energy
by acceleration from the electric field, surplus energy is released as light. The energy of this light is so high that this method is an effective way of generating ultraviolet light
.
When the recombination does not occur, further ionization proceeds by collision between high-energy electrons and a parent atom (molecule). Consequently, a multivalent ion is created and this ion is collapsed by Coulomb repulsion. This is called Coulomb explosion
.
As the Coulomb potential barrier is lowered, its width decreases, and this in turn increases the probability of electron tunneling through the barrier. The ADK probability formula gives the expression for the probability of tunnel ionization.
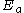 is the characteristic atomic electric field (the electric field at the bohr radius
is the characteristic atomic electric field (the electric field at the bohr radius
)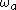 is the frequency associated with the ionization energy (i.e. the ground state energy)
is the frequency associated with the ionization energy (i.e. the ground state energy)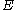 is the applied electric field
is the applied electric field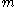 is the mass of an electron
is the mass of an electron
 is the electron charge
is the electron charge
 is the dirac constant
is the dirac constant
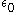 is the dielectric constant
is the dielectric constant
in an electrostatic (DC) field was solved schematically by Landau
. This provides a simplified physical system that given it proper exponential dependence of the ionization rate on the applied external field. When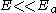 , the ionization rate for this system is given by:
, the ionization rate for this system is given by:
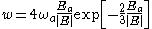
Landau expressed this in units where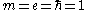 . In SI units
. In SI units
the previous parameters can be expressed as:
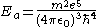 ,
,
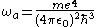 .
.
The ionization rate is the total probability current
through the outer classical turning point. This is found using the WKB approximation to match the ground state hydrogen wavefunction though the suppressed coulomb potential barrier.
by A.D.K..
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s in an atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
(or a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
) pass through the potential barrier and escape from the atom (or molecule). In an intense electric field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
, the potential barrier of an atom (molecule) is distorted drastically. Therefore, the length of the barrier that electrons have to pass decreases and electrons can escape from the atom (molecule) easily.
As an electric field of light is an alternating electric field, the direction of the electric field reverses after the half period of the field. Because electrons have a charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
, electrons escaping by tunnel ionization come and go to the atom (molecule) in every half period.
In this process, some electrons recombine with the nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
(nuclei). Because the electrons have gained a large quantity of kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
by acceleration from the electric field, surplus energy is released as light. The energy of this light is so high that this method is an effective way of generating ultraviolet light
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
.
When the recombination does not occur, further ionization proceeds by collision between high-energy electrons and a parent atom (molecule). Consequently, a multivalent ion is created and this ion is collapsed by Coulomb repulsion. This is called Coulomb explosion
Coulomb explosion
A Coulomb explosion is a mechanism for coupling electronic excitation energy from intense electromagnetic fields into atomic motion. The atomic motion can break the bonds that hold solids together...
.
Physical Process
Tunneling Ionization is a QM phenomenon; a non-zero probability event for observing a particle escaping from the deformed Coulomb potential barrier, obviously this phenomenon is forbidden by classical laws, as in the classical picture an electron does not have sufficient energy to escape.As the Coulomb potential barrier is lowered, its width decreases, and this in turn increases the probability of electron tunneling through the barrier. The ADK probability formula gives the expression for the probability of tunnel ionization.
Notation
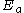 is the characteristic atomic electric field (the electric field at the bohr radius
is the characteristic atomic electric field (the electric field at the bohr radiusBohr radius
The Bohr radius is a physical constant, approximately equal to the most probable distance between the proton and electron in a hydrogen atom in its ground state. It is named after Niels Bohr, due to its role in the Bohr model of an atom...
)
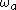 is the frequency associated with the ionization energy (i.e. the ground state energy)
is the frequency associated with the ionization energy (i.e. the ground state energy)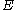 is the applied electric field
is the applied electric field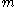 is the mass of an electron
is the mass of an electronElectron rest mass
The electron rest mass is the mass of a stationary electron. It is one of the fundamental constants of physics, and is also very important in chemistry because of its relation to the Avogadro constant...
 is the electron charge
is the electron chargeElementary charge
The elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
 is the dirac constant
is the dirac constantPlanck constant
The Planck constant , also called Planck's constant, is a physical constant reflecting the sizes of energy quanta in quantum mechanics. It is named after Max Planck, one of the founders of quantum theory, who discovered it in 1899...
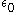 is the dielectric constant
is the dielectric constantPermittivity
In electromagnetism, absolute permittivity is the measure of the resistance that is encountered when forming an electric field in a medium. In other words, permittivity is a measure of how an electric field affects, and is affected by, a dielectric medium. The permittivity of a medium describes how...
DC Tunneling Ionization
Tunneling ionization from the ground state of a Hydrogen atomHydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively-charged proton and a single negatively-charged electron bound to the nucleus by the Coulomb force...
in an electrostatic (DC) field was solved schematically by Landau
Lev Landau
Lev Davidovich Landau was a prominent Soviet physicist who made fundamental contributions to many areas of theoretical physics...
. This provides a simplified physical system that given it proper exponential dependence of the ionization rate on the applied external field. When
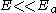 , the ionization rate for this system is given by:
, the ionization rate for this system is given by: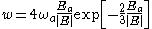
Landau expressed this in units where
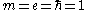 . In SI units
. In SI unitsInternational System of Units
The International System of Units is the modern form of the metric system and is generally a system of units of measurement devised around seven base units and the convenience of the number ten. The older metric system included several groups of units...
the previous parameters can be expressed as:
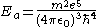 ,
,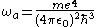 .
.The ionization rate is the total probability current
Probability current
In quantum mechanics, the probability current is a mathematical quantity describing the flow of probability density. Intuitively; if one pictures the probability density as an inhomogeneous fluid, then the probability current is the rate of flow of this fluid...
through the outer classical turning point. This is found using the WKB approximation to match the ground state hydrogen wavefunction though the suppressed coulomb potential barrier.
AC Electric Field
The ionization rate of an hydrogen atom in an alternating electric field, like that of a laser, can be treated, in the appropriate limit, as the DC ionization rate averaged over a single period of the electric fields oscillation. This models was proposed by Keldysh and then later expanded to hydrogen-like atomHydrogen-like atom
A hydrogen-like ion is any atomic nucleus with one electron and thus is isoelectronic with hydrogen. Except for the hydrogen atom itself , these ions carry the positive charge e, where Z is the atomic number of the atom. Examples of hydrogen-like ions are He+, Li2+, Be3+ and B4+...
by A.D.K..

