
Baylis–Hillman reaction
Encyclopedia
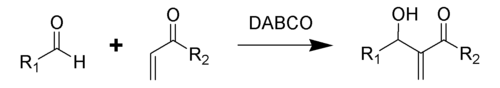
The Baylis–Hillman reaction is an organic reaction
of an aldehyde
and an α,β-unsaturated
electron-withdrawing group catalyzed by DABCO
(1,4-diazabicyclo[2.2.2]octane) to give an allyl
ic alcohol
. This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman. The Baylis–Hillman reaction, in the present day version, is an atom-economic
carbon
-carbon
bond formation reaction.
 In addition to DABCO, additional nucleophilic amine
In addition to DABCO, additional nucleophilic amine
s such as DMAP and DBU
as well as phosphines have been found to successfully catalyze this reaction.
Several reviews have been written.
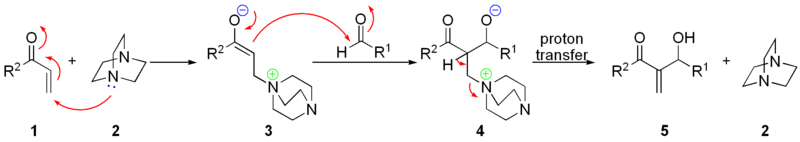
of DABCO 2 onto the α,β-unsaturated ketone 1 gives a zwitterionic intermediate 3, which will add to the electrophilic aldehyde
producing the keto-alcohol 4. Elimination
of the DABCO gives the desired allylic alcohol 5.
 A simple relationship exists between pKa
A simple relationship exists between pKa
of the base (as its conjugate acid
s) and the reaction rate
with quinuclidine
even more effective than DABCO. Protic
additives like methanol
, triethanolamine
, formamide
, and water also accelerate the reaction .
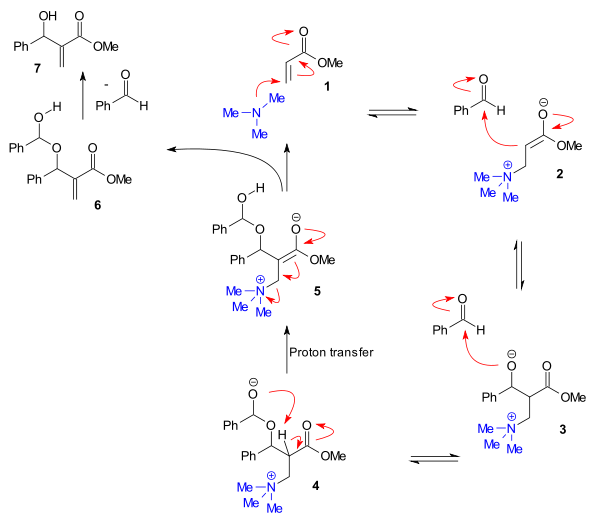
An alternative mechanism, based on extensive rate data, has been proposed for some aldehydes.. This mechanism (figure below) takes into account experimentally determined second order kinetics for the aldehyde and a substantial kinetic isotope effect
for the enone alpha-proton. In it a second aldehyde molecule reacts to form a hemiacetal
(4) and this step is followed by a rate-determining
proton transfer step to intermediate 5.
 In silico
In silico
experiments confirm this mechanism and also explain how protic additives increase reaction rates by facilitating the proton transfer step. Several of the key intermediates can also be detected experimentally with ESI-MS
A related reaction actually predating the Baylis–Hillman reaction utilising phosphines and not DABCO is the lesser known Rauhut–Currier reaction.
s and sp2 hybridized carbon electrophiles such as aldehydes, ketones and aldimine
s catalyzed by a nucleophile
. Under special reaction conditions the reaction is also found to extend to alkyl halides as the electrophilic reagent . In this variation amine nucleophiles are unsuitable and trialkyl phosphines are used instead. Under the given reaction conditions these phosphines do not react directly with the alkyl halide. The added base in the second step of this reaction promotes the elimination reaction
to the enone
.
 In the aza-Baylis–Hillman reaction the electrophile is an imine
In the aza-Baylis–Hillman reaction the electrophile is an imine
.
Recently Namboothiri and Deb developed a novel methodology for the synthesis of substituted α-hydroxyalkylated nitroalkenes by using MBH strategy under DMAP/MeCN and Imidazole/THF catalytic condition. Various activated carbonyl compounds like ethylglyoxylate, pyruvic aldehyde, trifluoromethyl pyruvate, diethylketomalonate were used as electrophiles and various aromatic, heteroaromatic, conjugated nitroalkenes were used as MBH substrates.
The Baylis–Hillman adducts and their derivatives have been extensively utilized for the generation of heterocycles
and other cyclic frameworks.
and DABCO
in DMF
is not limited to the monoadduct because the MBH adduct reacts with a second molecule of phenyl vinyl ketone in a nucleophilic conjugate addition
.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
of an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
and an α,β-unsaturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
electron-withdrawing group catalyzed by DABCO
DABCO
DABCO or 1,4-diazabicyclo[2.2.2]octane is a chemical compound. It is a polyurethane and Baylis-Hillman reaction catalyst, complexing ligand and Lewis base. It is used to regulate the reaction rate in Flexplay time-limited DVDs by adjusting pH. Antioxidants, like DABCO, are used to improve the...
(1,4-diazabicyclo[2.2.2]octane) to give an allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
ic alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman. The Baylis–Hillman reaction, in the present day version, is an atom-economic
Atom economy
Atom economy describes the conversion efficiency of a chemical process in terms of all atoms involved . In an ideal chemical process, the amount of starting materials or reactants equals the amount of all products generated and no atom is wasted...
carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
bond formation reaction.

Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s such as DMAP and DBU
DBU (chemistry)
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst and complexing ligand and a strong non-nucleophilic base.It is used as a curing agent for epoxy; it is used as a protecting...
as well as phosphines have been found to successfully catalyze this reaction.
Several reviews have been written.
Reaction mechanism
The nucleophilic additionNucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
of DABCO 2 onto the α,β-unsaturated ketone 1 gives a zwitterionic intermediate 3, which will add to the electrophilic aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
producing the keto-alcohol 4. Elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of the DABCO gives the desired allylic alcohol 5.

PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of the base (as its conjugate acid
Conjugate acid
Within the Brønsted–Lowry acid-base theory , a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton. A conjugate acid can also be seen as the chemical substance that releases, or donates, a proton in the forward chemical...
s) and the reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
with quinuclidine
Quinuclidine
Quinuclidine is an organic compound and a bicyclic amine and used as a catalyst and a chemical building block. It is a strong base with pKa of the conjugate acid of 11.0. This is due to greater availability of the nitrogen lone pair...
even more effective than DABCO. Protic
Protic
Protić is a Serbian surname. It may refer to:* Milorad B. Protić, an astronomer* Miodrag B. Protić, a painter* Stojan Protić, Yugoslav political figure...
additives like methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, triethanolamine
Triethanolamine
Triethanolamine, often abbreviated as TEA, is an organic chemical compound which is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Like other amines, triethanolamine is a strong base due to the lone pair of electrons on the nitrogen atom. Triethanolamine can...
, formamide
Formamide
Formamide, also known as methanamide, is an amide derived from formic acid. It is a clear liquid which is miscible with water and has an ammonia-like odor. It is used primarily for manufacturing sulfa drugs and synthesizing vitamins and as a softener for paper and fiber...
, and water also accelerate the reaction .
An alternative mechanism, based on extensive rate data, has been proposed for some aldehydes.. This mechanism (figure below) takes into account experimentally determined second order kinetics for the aldehyde and a substantial kinetic isotope effect
Kinetic isotope effect
The kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
for the enone alpha-proton. In it a second aldehyde molecule reacts to form a hemiacetal
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
(4) and this step is followed by a rate-determining
Rate-determining step
The rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In...
proton transfer step to intermediate 5.

In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
experiments confirm this mechanism and also explain how protic additives increase reaction rates by facilitating the proton transfer step. Several of the key intermediates can also be detected experimentally with ESI-MS
A related reaction actually predating the Baylis–Hillman reaction utilising phosphines and not DABCO is the lesser known Rauhut–Currier reaction.
Scope
The MBH reaction in general is any reaction of electron deficient alkeneAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s and sp2 hybridized carbon electrophiles such as aldehydes, ketones and aldimine
Aldimine
In organic chemistry, an aldimine is an imine that is an analog of an aldehyde.As such, aldimines have the general formula R–CH=N–R. Aldimines are similar to ketimines, which are analogs of ketones.An important subset of aldimines are the Schiff bases,...
s catalyzed by a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
. Under special reaction conditions the reaction is also found to extend to alkyl halides as the electrophilic reagent . In this variation amine nucleophiles are unsuitable and trialkyl phosphines are used instead. Under the given reaction conditions these phosphines do not react directly with the alkyl halide. The added base in the second step of this reaction promotes the elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
to the enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
.

Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
.
Recently Namboothiri and Deb developed a novel methodology for the synthesis of substituted α-hydroxyalkylated nitroalkenes by using MBH strategy under DMAP/MeCN and Imidazole/THF catalytic condition. Various activated carbonyl compounds like ethylglyoxylate, pyruvic aldehyde, trifluoromethyl pyruvate, diethylketomalonate were used as electrophiles and various aromatic, heteroaromatic, conjugated nitroalkenes were used as MBH substrates.
The Baylis–Hillman adducts and their derivatives have been extensively utilized for the generation of heterocycles
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
and other cyclic frameworks.
Limitations
The MBH reaction of phenyl vinyl ketone with benzaldehydeBenzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
and DABCO
DABCO
DABCO or 1,4-diazabicyclo[2.2.2]octane is a chemical compound. It is a polyurethane and Baylis-Hillman reaction catalyst, complexing ligand and Lewis base. It is used to regulate the reaction rate in Flexplay time-limited DVDs by adjusting pH. Antioxidants, like DABCO, are used to improve the...
in DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
is not limited to the monoadduct because the MBH adduct reacts with a second molecule of phenyl vinyl ketone in a nucleophilic conjugate addition
Nucleophilic conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special...
.

