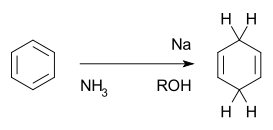
Birch reduction
Encyclopedia
The Birch Reduction is an organic reaction which is particularly useful in synthetic organic chemistry. The reaction was reported in 1944 by the Australian chemist Arthur Birch (1915–1995) working in the Dyson Perrins Laboratory
in the University of Oxford
, building on earlier work by Wooster and Godfrey in 1937. It converts aromatic compounds
having a benzenoid ring into a product, 1,4-cyclohexadienes
, in which two hydrogen atoms have been attached on opposite ends of the molecule. It is the organic reduction of aromatic rings in liquid ammonia
with sodium
, lithium
or potassium
and an alcohol
, such as ethanol
and tert-butanol
. This reaction is quite unlike catalytic hydrogenation
, which usually reduces the aromatic ring all the way to a cyclohexane
.
The original reaction reported by Arthur Birch in 1944 utilized sodium
and ethanol
. Subsequently A. L. Wilds noted that better yields result with lithium. Also the use of t-butyl alcohol has become common. The reaction is one of the main organic reactions utilized in all types of syntheses.
An example is the reduction of naphthalene
:
Several reviews have been published.
salt [Na(NH3)x]+ e−, associated with the intense blue color of these solutions. The solvated electron
s add to the aromatic ring to give a radical anion
. The added alcohol supplies a proton to the radical anion and also to the penultimate carbanion; for most substrates ammonia is not acidic enough.
Where the radical-anion is protonated initially determines the structure of the product. With an electron donor as methoxy (MeO) or alkyl protonation has been thought by some investigators as being ortho (i.e. adjacent or 1,2) to the substituent. Other investigators have thought the protonation is meta (1,3) to the substituent. Arthur Birch favored meta protonation. With electron withdrawing substituents protonation has been thought to come at the site (ipso) of the substituent or para (1,4). Again, there has been varied opinion. A. J. Birch’s empirical rules say that for the donor substituents the final product has the maximum number of substituents on the final double bonds. For electron withdrawing groups the double bonds of the product have avoided the substituents. The placement preference of groups in the mechanism and in the final product is termed regioselectivity.
which are taken up by the aromatic ring to form the corresponding radical anion B in the first step of the reaction. This is followed by protonation by the alcohol to form a cyclohexadienyl radical C. Next, a second electron is transferred to the radical to form a cyclohexadienyl carbanion D. In the last step a second proton leads the cyclohexadienyl carbanion to the unconjugated cyclohexadienyl product. These steps are outlined below for the case of anisole.
The reaction is known to be third order
– first order in aromatic, first order in the alkali metal, and first order in the alcohol.
This requires the rate-limiting step to be the conversion of radical anion B to the cyclohexadienyl radical C.
and are considered below. Birch’s rule for aromatics with electron donors such as methoxyl or alkyl is that the product will have the residual double bonds bearing the maximum number of substituents. For aromatics with electron withdrawing groups such as carboxyl, the substituent groups avoid the double bonds. In both cases, with electron donating and with withdrawing groups, the residual double bonds are unconjugated (vide infra). It has been a matter of intense interest to understand reaction mechanisms accounting for this regioselectivity. The essential features are:
, resulting from the addition of an electron, would become highest meta to an electron donor (such as methoxy or methyl) due to avoiding the usual ortho-para high density in the neutral species.
computations in 1961 it was shown that the Birch mechanism was incorrect. The correct mechanism O is depicted below."Orientation in Metal Ammonia Reductions," Zimmerman, H. E, Tetrahedron, 1961, 16, 169-176.
The two a-priori alternative mechanisms O and M:
Bothner-By in 1959 had given qualitative arguments favoring meta-protonation as had been suggested previously by Birch.
Burnham in 1969 concluded that protonation is unlikely to occur predominantly at the ortho position and the reaction most probably occurs at the meta position but may occur at both sites at similar rates.Burnham, D. R., Tetrahedron, 1969 25, 897-904
Subsequently, Birch in a review article noted that no experimental method at the time existed which would determine which was correct. But he did note that publication by Burnham favored meta attack.
In 1980 publications Birch collaborated with Leo Radom and considered ortho and meta
densities to be close with a slight ortho preference but with mixtures of ortho and meta protonation occurring. RHF/sto-3g and UHF/sto-3g computations were used to conclude that both ortho and meta substitutions would occur with a slight preference for ortho.
Thus there had been a decade of controversy in the literature in which each of these two possible mechanisms was considered to be correct.
in protonation in a protium–deuterium medium would be greater for the radical anion, of the first protonation step, than for the carbanion of the penultimate step. The reasoning was that carbanions are much more basic than the corresponding radical anions and thus will react more exothermically and less selectively in protonation. Experimentally it was determined that less deuterium at the ortho site than meta resulted (1:7) for a variety of methoxylated aromatics. This is a consequence of the greater selectivity of the radical anion protonation. Computations (e.g. ROHF/6-31g) of the electron densities concurred with the experimental observations. Also, it was ascertained that frontier orbital densities did not, and these had been used in some previous reports.
Subsequently, in 1992 and 1996 Birch published twice still suggesting that meta protonation was preferred. This was a reversal of his earlier views as published with Leo Radom.
However, textbooks, publishing on the mechanism of the Birch Reduction, have noted that ortho protonation of the initial radical anion is preferred.
Mechanism of reduction of benzoic acids, including possible alkylation
This dianion results independent of whether alcohol is used in the reduction or not. Thus the initial protonation by t-butyl alcohol or ammonia is para rather than ipso as seen in the step from B to C.
cyclohexadienes also poses mechanistic questions. Thus as shown in the figure below there are three resonance structures B, C and D for the carbanion. Simple Hückel computations lead, as noted in the first entry of the table below, to equal electron densities at the three atoms 1, 3 and 5. However, in contrast to densities the Hückel computation is less naïve about bond order
s, and bonds 2-3 and 5-6 will be shortened as shown in the first entry of the table. With bond orders modifying simple exchange integrals in a Mulliken-Wheland-Mann computation it was shown that electron density at the central atom 1 become largest. More modern RHF computations lead to the same result.
Electron introduction to benzene and 3 resonance structures for the carbanion of the second step, and central protonation to give the unconjugated diene:
Five carbons of the cyclohexadienyl anion.
There are known precedents for central anion protonation. Thus conjugated enolates as C=C-C=C-O- have been known for some time as kinetically protonating in the center of the enolate system to afford the β,γ-unsaturated carbonyl compound under conditions where the anion, and not the enol, is the species protonated.
can also undergo nucleophilic substitution
with carbon-carbon bond
formation. In substituted aromatic compounds an electron-withdrawing substituent, such as a carboxylic acid
, stabilizes a carbanion
and the least-substituted olefin is generated. With an electron-donating substituent the opposite effect is obtained. The reaction produces more of the less thermodynamically stable non-conjugated 1,4-addition product than the more stable conjugated
1,3-diene because the largest orbital coefficient of the HOMO of the conjugated pentadienyl anion intermediate is on the central carbon atom. Once formed, the resulting 1,4-cyclohexadiene is unable to equilibrate to the thermodynamically more stable product; therefore, the observed kinetic product is produced. Experimental alkali metal alternatives that are safer to handle, such as the M-SG reducing agent
, also exist.
In Birch alkylation the anion formed in the Birch reduction is trapped by a suitable electrophile
such as a haloalkane
, for example:
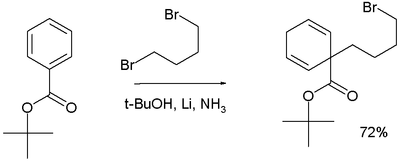
In the reaction depicted below, 1,4-dibromobutane is added to t-butyl benzoate to form an alkylated 1,4-cyclohexadiene product:
as well as a mixture of n-propylamine
and ethylenediamine, both with comparable results. The latter one actually is a modification of the Benkeser Reaction
, which in its original forms tends to reduce naphthalene all the way to octahydro- and decahydronaphthalene.
This reduction of naphthalene to isotetralin (1,4,5,8-tetrahydronaphthalene) produces some tetralin
(1,2,3,4-tetrahydronaphthalene) as byproduct, as is the case with the regular Birch reduction.
Dyson Perrins Laboratory
The Dyson Perrins Laboratory in the science area of the University of Oxford was the main centre for research into organic chemistry of the University from its foundation in 1916 until its closure as a laboratory in 2003...
in the University of Oxford
University of Oxford
The University of Oxford is a university located in Oxford, United Kingdom. It is the second-oldest surviving university in the world and the oldest in the English-speaking world. Although its exact date of foundation is unclear, there is evidence of teaching as far back as 1096...
, building on earlier work by Wooster and Godfrey in 1937. It converts aromatic compounds
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
having a benzenoid ring into a product, 1,4-cyclohexadienes
1,4-Cyclohexadiene
1,4-Cyclohexadiene is a highly flammable cycloalkene that occurs as a colorless clear liquid.1,4-Cyclohexadiene and related compounds may be prepared from benzene using lithium or sodium in liquid ammonia, this process being known as a Birch reduction. However 1,4-cyclohexadiene is easily oxidised...
, in which two hydrogen atoms have been attached on opposite ends of the molecule. It is the organic reduction of aromatic rings in liquid ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
with sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
or potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
and an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
, such as ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
and tert-butanol
Tert-Butanol
tert-Butanol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether...
. This reaction is quite unlike catalytic hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
, which usually reduces the aromatic ring all the way to a cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
.
The original reaction reported by Arthur Birch in 1944 utilized sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
. Subsequently A. L. Wilds noted that better yields result with lithium. Also the use of t-butyl alcohol has become common. The reaction is one of the main organic reactions utilized in all types of syntheses.
An example is the reduction of naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
:
Several reviews have been published.
Basic reaction mechanism
A solution of sodium in liquid ammonia consists of the electrideElectride
An electride is an ionic compound in which an electron is the anion. The first electrides to be studied in depth were solutions of alkali metals in ammonia. When sodium metal dissolves in ammonia, the result is a blue solution consisting of [Na6]+ and solvated electrons. Such solutions are powerful...
salt [Na(NH3)x]+ e−, associated with the intense blue color of these solutions. The solvated electron
Solvated electron
A solvated electron is a free electron in a solution. Solvated electrons occur widely although they are often not observed directly. The deep colour of solutions of alkali metals in ammonia arises form the presence of solvated electrons: blue when dilute and copper-colored when more concentrated...
s add to the aromatic ring to give a radical anion
Radical ion
A radical ion is a free radical species that carries a charge. Radical ions are encountered in organic chemistry as reactive intermediates and in mass spectrometry as gas phase ions...
. The added alcohol supplies a proton to the radical anion and also to the penultimate carbanion; for most substrates ammonia is not acidic enough.
Regioselectivity
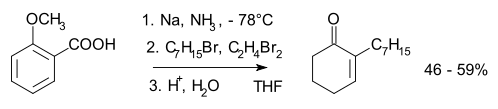
The reduction of anisole is one of the simplest examples and is shown in Eqn. 1. Still another example is that of benzoic acid illustrated in Eqn. 2.Where the radical-anion is protonated initially determines the structure of the product. With an electron donor as methoxy (MeO) or alkyl protonation has been thought by some investigators as being ortho (i.e. adjacent or 1,2) to the substituent. Other investigators have thought the protonation is meta (1,3) to the substituent. Arthur Birch favored meta protonation. With electron withdrawing substituents protonation has been thought to come at the site (ipso) of the substituent or para (1,4). Again, there has been varied opinion. A. J. Birch’s empirical rules say that for the donor substituents the final product has the maximum number of substituents on the final double bonds. For electron withdrawing groups the double bonds of the product have avoided the substituents. The placement preference of groups in the mechanism and in the final product is termed regioselectivity.
Overall details of the reaction mechanism
The solution of metal in ammonia provides electronsElectride
An electride is an ionic compound in which an electron is the anion. The first electrides to be studied in depth were solutions of alkali metals in ammonia. When sodium metal dissolves in ammonia, the result is a blue solution consisting of [Na6]+ and solvated electrons. Such solutions are powerful...
which are taken up by the aromatic ring to form the corresponding radical anion B in the first step of the reaction. This is followed by protonation by the alcohol to form a cyclohexadienyl radical C. Next, a second electron is transferred to the radical to form a cyclohexadienyl carbanion D. In the last step a second proton leads the cyclohexadienyl carbanion to the unconjugated cyclohexadienyl product. These steps are outlined below for the case of anisole.
The reaction is known to be third order
Order of reaction
In chemical kinetics, the order of reaction with respect to certain reactant, is defined as the power to which its concentration term in the rate equation is raised .For example, given a chemical reaction 2A + B → C with a rate equation...
– first order in aromatic, first order in the alkali metal, and first order in the alcohol.
This requires the rate-limiting step to be the conversion of radical anion B to the cyclohexadienyl radical C.
Reaction regioselectivity
Birch Reduction has several intricate mechanistic features. These features govern the reaction’s regioselectivityRegioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
and are considered below. Birch’s rule for aromatics with electron donors such as methoxyl or alkyl is that the product will have the residual double bonds bearing the maximum number of substituents. For aromatics with electron withdrawing groups such as carboxyl, the substituent groups avoid the double bonds. In both cases, with electron donating and with withdrawing groups, the residual double bonds are unconjugated (vide infra). It has been a matter of intense interest to understand reaction mechanisms accounting for this regioselectivity. The essential features are:
- In liquid ammonia alkali metals dissolve to give a blue solution thought of simplistically as having “free electrons”. The electrons are taken up by the aromatic ring, one at a time. Once the first electron has been absorbed, a radical-anion has been formed. Next the alcohol molecule donates its hydroxylic hydrogen to form a new C-H bond; at this point a radicalRadical (chemistry)Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
has been formed. This is followed by the second electron being picked up to give a carbanionCarbanionA carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
of the cyclohexadienyl type (i.e. with C=C-C-C=C in a six-ring and charged minus ). Then this cyclohexadienyl anion is protonatedProtonationIn chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
by the alcohol present. The protonation takes place in the middle of the cyclohexadienyl system. This (regio-)selectivity is unique and characteristic.
- Where the radical-anion is protonated initially determines the structure of the product. With an electron donor as methoxy (MeO) or alkyl protonation has been thought by some investigators as being ortho (i.e. adjacent or 1,2) to the substituent. Other investigators have thought the protonation is meta (1,3) to the substituent. Arthur Birch favored meta protonation. With electron withdrawing substituents protonation has been thought to come at the site (ipso) of the substituent or para (1,4). Again, there has been varied opinion. A. J. Birch’s empirical rules say that for the donor substituents the final product has the maximum number of substituents on the final double bonds. For electron withdrawing groups the double bonds of the product have avoided the substituents. The placement preference of groups in the mechanism and in the final product is termed regioselectivity.
- The reaction mechanism provides the details of molecular change as a reaction proceeds. In the case of donating groups A. J. Birch's preference for meta protonation of the radical anion was based on qualitative reasoning. And it had been noted that no experimental test of this was known.
- In 1961 a simple computation of the electron densities of the radical anion revealed that it was the ortho site which was most negative and thus most likely to protonate. However, A. J. Birch seemed to overlook this result. Additionally, the second proton had been determined by the computations to occur in the center of the cyclohexadienyl anion to give an unconjugated product.
- Of historical interest is the uncertainty in the chemical literature at this point. Indeed, there were some further computational results reported. These varied from suggesting a preference for meta radical-anion protonation to suggesting a mixture of ortho and meta protonation.
- In 1990 and 1993 an esoteric test was devised which showed that ortho protonation of the radical anion was preferred over meta (seven to one). This was accompanied by more modern computation which concurred. Both experiment and computations were in agreement with the early 1961 computations.
- With electron withdrawing groups there are literature examples demonstrating the nature of the carbanion just before final protonation. This revealed that the initial radical-anion protonation occurs para to the withdrawing substituent.
- The remaining item for discussion is the final protonation of the cyclohexadienyl anion. In 1961 it was found that simple Hückel computations were unable to distinguish between the different protonation sites. However, when the computations were modified with somewhat more realistic assumptions, the Hückel computations revealed the center carbon to the preferred. The more modern 1990 and 1993 computations were in agreement.
Underlying mechanism of the Birch reduction
The original Birch mechanism suggested that the initial radical anion protonation was meta to the ring methoxy and alkyl groups and the last step, protonation of a cyclohexadienyl anion, was ortho. Birch’s original mechanism was based on qualitative reasoning, namely that the radical anion’s electron densityElectron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
, resulting from the addition of an electron, would become highest meta to an electron donor (such as methoxy or methyl) due to avoiding the usual ortho-para high density in the neutral species.
Hückel computations
Using simple HückelHückel method
The Hückel method or Hückel molecular orbital method proposed by Erich Hückel in 1930, is a very simple linear combination of atomic orbitals molecular orbitals method for the determination of energies of molecular orbitals of pi electrons in conjugated hydrocarbon systems, such as ethene,...
computations in 1961 it was shown that the Birch mechanism was incorrect. The correct mechanism O is depicted below."Orientation in Metal Ammonia Reductions," Zimmerman, H. E, Tetrahedron, 1961, 16, 169-176.
The two a-priori alternative mechanisms O and M:
Subsequent literature and varying views
However, Birch did not accept this conclusion and continued publications suggesting meta protonation of the radical anion. He suggested the meta attack results from “opposition of the ortho and para initial charge”.Bothner-By in 1959 had given qualitative arguments favoring meta-protonation as had been suggested previously by Birch.
Burnham in 1969 concluded that protonation is unlikely to occur predominantly at the ortho position and the reaction most probably occurs at the meta position but may occur at both sites at similar rates.Burnham, D. R., Tetrahedron, 1969 25, 897-904
Subsequently, Birch in a review article noted that no experimental method at the time existed which would determine which was correct. But he did note that publication by Burnham favored meta attack.
In 1980 publications Birch collaborated with Leo Radom and considered ortho and meta
densities to be close with a slight ortho preference but with mixtures of ortho and meta protonation occurring. RHF/sto-3g and UHF/sto-3g computations were used to conclude that both ortho and meta substitutions would occur with a slight preference for ortho.
Thus there had been a decade of controversy in the literature in which each of these two possible mechanisms was considered to be correct.
Experimental testing and computational verification
Then in 1990 and 1993 a method was finally devised to experimentally assess whether the anisole and toluene radical anion protonated ortho or meta. The esoteric method began with the premise that the isotope selectivityKinetic isotope effect
The kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
in protonation in a protium–deuterium medium would be greater for the radical anion, of the first protonation step, than for the carbanion of the penultimate step. The reasoning was that carbanions are much more basic than the corresponding radical anions and thus will react more exothermically and less selectively in protonation. Experimentally it was determined that less deuterium at the ortho site than meta resulted (1:7) for a variety of methoxylated aromatics. This is a consequence of the greater selectivity of the radical anion protonation. Computations (e.g. ROHF/6-31g) of the electron densities concurred with the experimental observations. Also, it was ascertained that frontier orbital densities did not, and these had been used in some previous reports.
Subsequently, in 1992 and 1996 Birch published twice still suggesting that meta protonation was preferred. This was a reversal of his earlier views as published with Leo Radom.
However, textbooks, publishing on the mechanism of the Birch Reduction, have noted that ortho protonation of the initial radical anion is preferred.
Birch reduction with electron withdrawing substituents
In contrast to the examples with electron donating substituents, the case with withdrawing groups is more readily obvious. Thus, as depicted below, the structure of the penultimate dianion D is characterized by its being subject to trapping by alkyl halides.Mechanism of reduction of benzoic acids, including possible alkylation
This dianion results independent of whether alcohol is used in the reduction or not. Thus the initial protonation by t-butyl alcohol or ammonia is para rather than ipso as seen in the step from B to C.
Second step of the Birch reduction with regiochemistry giving unconjugated cyclohexadienes
The second step of the Birch reduction affording unconjugatedConjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
cyclohexadienes also poses mechanistic questions. Thus as shown in the figure below there are three resonance structures B, C and D for the carbanion. Simple Hückel computations lead, as noted in the first entry of the table below, to equal electron densities at the three atoms 1, 3 and 5. However, in contrast to densities the Hückel computation is less naïve about bond order
Bond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
s, and bonds 2-3 and 5-6 will be shortened as shown in the first entry of the table. With bond orders modifying simple exchange integrals in a Mulliken-Wheland-Mann computation it was shown that electron density at the central atom 1 become largest. More modern RHF computations lead to the same result.
Electron introduction to benzene and 3 resonance structures for the carbanion of the second step, and central protonation to give the unconjugated diene:
Five carbons of the cyclohexadienyl anion.
| Approximation | Density Atom 3 | Density Atom 2 | Density Atom 1 | Bond Order 2-3 | Bond Order 1-2 |
|---|---|---|---|---|---|
| Hückel (1st Approx) | 0.333 | 0.00 | 0.333 | 0.788 | 0.578 |
| 2nd Approx | 0.317 | 0.00 | 0.365 | 0.802 | 0.564 |
| 3rd Approx | 0.316 | 0.00 | 0.368 | 0.802 | 0.562 |
There are known precedents for central anion protonation. Thus conjugated enolates as C=C-C=C-O- have been known for some time as kinetically protonating in the center of the enolate system to afford the β,γ-unsaturated carbonyl compound under conditions where the anion, and not the enol, is the species protonated.
Birch alkylation
In the presence of an alkyl halide the carbanionCarbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
can also undergo nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
with carbon-carbon bond
Carbon-carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is said to be formed between one hybridized orbital from each...
formation. In substituted aromatic compounds an electron-withdrawing substituent, such as a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
, stabilizes a carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
and the least-substituted olefin is generated. With an electron-donating substituent the opposite effect is obtained. The reaction produces more of the less thermodynamically stable non-conjugated 1,4-addition product than the more stable conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
1,3-diene because the largest orbital coefficient of the HOMO of the conjugated pentadienyl anion intermediate is on the central carbon atom. Once formed, the resulting 1,4-cyclohexadiene is unable to equilibrate to the thermodynamically more stable product; therefore, the observed kinetic product is produced. Experimental alkali metal alternatives that are safer to handle, such as the M-SG reducing agent
M-SG reducing agent
In M-SG an alkali metal is absorbed into silica gel at elevated temperatures. The resulting black powder material is an effective reducing agent and safe to handle as opposed to the pure metal. The material can also be used as a desiccant and as a hydrogen source .The metal is either sodium or a...
, also exist.
In Birch alkylation the anion formed in the Birch reduction is trapped by a suitable electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
such as a haloalkane
Haloalkane
The haloalkanes are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and...
, for example:
In the reaction depicted below, 1,4-dibromobutane is added to t-butyl benzoate to form an alkylated 1,4-cyclohexadiene product:
Modifications of the Birch reduction
Since liquid ammonia has to be condensed into the flask and has to evaporate overnight after the reaction is complete, the whole procedure can be quite troublesome and time-consuming. However, alternative solvents have been employed, such as THFThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
as well as a mixture of n-propylamine
Propylamine
Propylamine, also known as n-propylamine, is an amine with the chemical formula C3H9N.Propylamine is a weak base with its Kb equaling 4.7 × 10-4.-Preparation:...
and ethylenediamine, both with comparable results. The latter one actually is a modification of the Benkeser Reaction
Benkeser Reaction
The Benkeser reduction reaction is the catalytic hydrogenation of polycyclic aromatic hydrocarbons, especially naphthalene using lithium or calcium metal and any of the following primary amines or low molecular weight alkyl groups as reductants: -CH3, -CH2CH3, -CH2CH2CH3, -CH2CH2NH2.This reaction...
, which in its original forms tends to reduce naphthalene all the way to octahydro- and decahydronaphthalene.
This reduction of naphthalene to isotetralin (1,4,5,8-tetrahydronaphthalene) produces some tetralin
Tetralin
Tetralin is a hydrocarbon having the chemical formula C10H12. This molecule is similar to the naphthalene chemical structure except that one ring is saturated.The compound can be synthesized in a Bergman cyclization...
(1,2,3,4-tetrahydronaphthalene) as byproduct, as is the case with the regular Birch reduction.