
Criegee rearrangement
Encyclopedia
The Criegee rearrangement is a rearrangement reaction
named after Rudolf Criegee
. In this organic reaction
a tertiary alcohol is cleaved in an organic oxidation by a peroxyacid to a ketone
. The acid used is often p-nitroperoxybenzoic acid because the p-nitrobenzoic acid anion is a good leaving group.
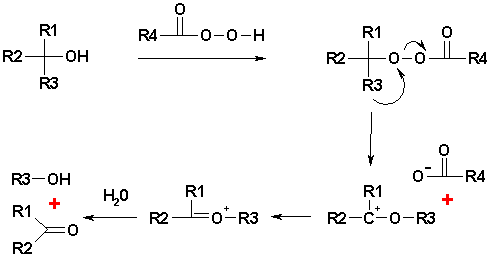
The reaction mechanism has similarities with the Baeyer-Villiger oxidation
where the intermediate hydroxyperacid is called a Criegee intermediate. The per-acid forms a per-ester with the alcohol group. One alkyl substituent migrates from carbon to the adjacent oxygen atom, replacing the carboxylic acid
leaving behind a carbocation
. A hydrolysis
step forms the ketone together with the alcohol. The order of migrationary aptitude is a tert-butyl as the best substituent
followed by isopropyl
then ethyl
and then the methyl group. From this it is inferred that the migrating group carries a partial positive charge in the transition state
leading to the carbocation. The consecutive Criegee Rearrangement is carried out in an acidic environment and an ester forms from the carbocation. This opens the way to multiple O-insertion reactions, eventually leading to the ortho ester. In the Criegee reaction a vicinal
diol
is cleaved by lead tetraacetate to the corresponding ketones and acetic acid
.
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
named after Rudolf Criegee
Rudolf Criegee
Rudolf Criegee was a German chemist. He studied in Tübingen, Greifswald, and Würzburg and received his doctorate at Würzburg in 1925. He proposed a reaction mechanism for ozonolysis in 1953. The Criegee rearrangement is named after him...
. In this organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
a tertiary alcohol is cleaved in an organic oxidation by a peroxyacid to a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
. The acid used is often p-nitroperoxybenzoic acid because the p-nitrobenzoic acid anion is a good leaving group.

The reaction mechanism has similarities with the Baeyer-Villiger oxidation
Baeyer-Villiger oxidation
The Baeyer–Villiger oxidation is an organic reaction in which a ketone is oxidized to an ester by treatment with peroxy acids or hydrogen peroxide. Key features of the Baeyer–Villiger oxidation are its stereospecificity and predictable regiochemistry...
where the intermediate hydroxyperacid is called a Criegee intermediate. The per-acid forms a per-ester with the alcohol group. One alkyl substituent migrates from carbon to the adjacent oxygen atom, replacing the carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
leaving behind a carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
. A hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
step forms the ketone together with the alcohol. The order of migrationary aptitude is a tert-butyl as the best substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
followed by isopropyl
Isopropyl
In organic chemistry, isopropyl is a propyl with a group attached to the secondary carbon. If viewed as a functional group an isopropyl is an organic compound with a propyl group attached at its secondary carbon.The bond is therefore on the middle carbon....
then ethyl
Ethyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
and then the methyl group. From this it is inferred that the migrating group carries a partial positive charge in the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
leading to the carbocation. The consecutive Criegee Rearrangement is carried out in an acidic environment and an ester forms from the carbocation. This opens the way to multiple O-insertion reactions, eventually leading to the ortho ester. In the Criegee reaction a vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
is cleaved by lead tetraacetate to the corresponding ketones and acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
.

