History of mass spectrometry
Encyclopedia
The history of mass spectrometry dates back more than one hundred years and has its roots in physical and chemical studies regarding the nature of matter. The study of gas discharges in the mid 19th century led to the discovery of anode
and cathode ray
s, which turned out to be positive ion
s and electron
s. Improved capabilities in the separation of these positive ions enabled the discovery of stable isotope
s of the elements. The first such discovery was with the atom neon
, which was shown by mass spectrometry
to have at least two stable isotopes: neon-20 with 10 proton
s and 10 neutron
s and neon-22 with 10 protons and 12 neutrons. Mass spectrometers were used in the Manhattan Project
for the separation of isotopes of uranium necessary to create the atomic bomb.
was an early 19th century attempt to explain the properties of the chemical element
s using the internal structure of the atom
. In 1815, the English chemist William Prout
observed that the atomic weight
s that had been measured were integer
multiples of the atomic weight of hydrogen
. Prout's hypothesis remained influential in chemistry throughout the 1820s. However, more careful measurements of the atomic weights, such as those compiled by Jöns Jakob Berzelius
in 1828 or Edward Turner in 1832, appeared to disprove it. In particular the atomic weight of chlorine
, which is 35.45 times that of hydrogen
, could not at the time be explained in terms of Prout's hypothesis. It would take the better part of a century for this problem to be resolved.
investigated the light emitted in discharge tubes and the influence of magnetic fields on the glow. Later, in 1869, Johann Wilhelm Hittorf
studied discharge tubes with energy rays extending from a negative electrode
, the cathode. These rays produced a fluorescence
when they hit a tube's glass walls, and when interrupted by a solid object they cast a shadow.
Canal rays, also called Anode rays, were observed by Eugen Goldstein
, in 1886. Goldstein used a gas discharge tube
which had a perforated cathode
. The rays are produced in the holes (canals) in the cathode and travels in a direction opposite to the "cathode ray
s," which are streams of electron
s. Goldstein called these positive rays "Kanalstrahlen" - canal rays. In 1907 a study of how this "ray" was deflected in a magnetic field
, revealed that the particles
making up the ray were not all the same mass
 In 1913, as part of his exploration into the composition of canal rays, J. J. Thomson
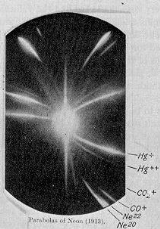
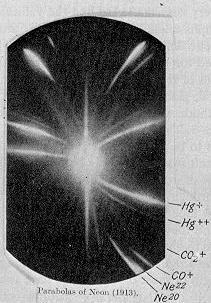
In 1913, as part of his exploration into the composition of canal rays, J. J. Thomson
channeled a stream of ionized neon through a magnetic and an electric field and measured its deflection by placing a photographic plate in its path. Thomson observed two patches of light on the photographic plate (see image on right), which suggested two different parabolas of deflection. Thomson concluded that the neon gas was composed of atoms of two different atomic masses (neon-20 and neon-22).
Thomson's student Francis William Aston
continued the research at the Trinity College, building the first full functional mass spectrometer in 1919. He was able to identify isotopes of chlorine
(35 and 37), bromine
(79 and 81), and krypton
(78, 80, 82, 83, 84 and 86), proving that these natural occurring elements are composed of a combination of isotopes. The use of electromagnetic focusing in mass spectrograph
which rapidly allowed him to identify no fewer than 212 of the 287 naturally occurring isotopes. In 1921 F. W. Aston became a fellow of the famous Royal Society
.
His work on isotopes also led to his formulation of the Whole Number Rule
which states that "the mass of the oxygen isotope being defined [as 16], all the other isotopes have masses that are very nearly whole numbers," a rule that was used extensively in the development of nuclear energy
. The exact mass of many isotopes was measured leading to the result that hydrogen has a 1% higher mass than expected by the average mass of the other elements. Aston speculated about the subatomic energy and the use of it in 1936.
In 1918, Arthur Jeffrey Dempster
developed the first modern mass spectrometer
, which was over 100 times more accurate than previous versions, and established the basic theory and design of mass spectrometers that is still used to this day. Dempster's research over his career centered around the mass spectrometer and its applications, leading in 1935 to his discovery of the uranium isotope 235U. This isotope's ability to cause a rapidly expanding fission
nuclear chain reaction
allowed the development of the atom bomb and nuclear power
.
In 1932, Kenneth Bainbridge
developed a mass spectrometer with a resolving power of 600 and a relative precision of one part in 10,000. He used this instrument to verify the equivalence of mass and energy
, E = mc2.
is a sector mass spectrometer
that was used for separating the isotopes of uranium
developed by Ernest O. Lawrence during the Manhattan Project
and was similar to the Cyclotron
invented by Lawrence. Its name is a concatenation
of Cal. U.-tron, in tribute to the University of California
, Lawrence's institution and the contractor of the Los Alamos
laboratory. They were implemented for industrial scale uranium enrichment at the Oak Ridge, Tennessee
Y-12 plant
established during the war and provided much of the uranium used for the "Little Boy
" nuclear weapon
, which was dropped onto Hiroshima
in 1945.
mass spectrometry was developed by Alan G. Marshall
and Melvin B. Comisarow at the University of British Columbia
in 1974. The inspiration was earlier developments in conventional ICR and Fourier Transform Nuclear Magnetic Resonance (FT-NMR) spectroscopy.
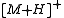 .
.
Chemical ionization was developed in the 1960s. Ionization of sample (analyte) is achieved by interaction of its molecules with reagent ions. The analyte is ionized by ion-molecule reactions during collisions in the source. The process may involve transfer of an electron, a proton or other charged species between the reactants. This is a less energetic procedure than electron ionization
and the ions produced are, for example, protonated molecules: [M + H]+. These ions are often relatively stable, tending not to fragment as readily as ions produced by electron ionization
.
Matrix-assisted laser desorption/ionization
(MALDI) is a soft ionization
technique used in mass spectrometry
, allowing the analysis of biomolecule
s (biopolymer
s such as proteins, peptides and sugars) and large organic
molecules (such as polymers, dendrimers and other macromolecules), which tend to be fragile and fragment when ionized by more conventional ionization methods. It is most similar in character to electrospray ionization
both in relative softness and the ions produced (although it causes much fewer multiply charged ions). The term was first used in 1985 by Franz Hillenkamp
, Michael Karas
and their colleagues. These researchers found that the amino acid
alanine
could be ionized more easily if it was mixed with the amino acid tryptophan
and irradiated with a pulsed 266 nm laser. The tryptophan was absorbing the laser energy and helping to ionize the non-absorbing alanine. Peptides up to the 2843 Da peptide melittin
could be ionized when mixed with this kind of “matrix”.
The breakthrough for large molecule laser desorption ionization came in 1987 when Koichi Tanaka
of Shimadzu Corp. and his co-workers used what they called the “ultra fine metal plus liquid matrix method” that combined 30 nm cobalt
particles in glycerol
with a 337 nm nitrogen laser
for ionization. Using this laser and matrix combination, Tanaka was able to ionize biomolecules as large as the 34,472 Da protein carboxypeptidase-A. Tanaka received one-quarter of the 2002 Nobel Prize in Chemistry
for demonstrating that, with the proper combination of laser wavelength and matrix, a protein can be ionized. Karas and Hillenkamp were subsequently able to ionize the 67 kDa protein albumin using a nicotinic acid matrix and a 266 nm laser. Further improvements were realized through the use of a 355 nm laser and the cinnamic acid
derivatives ferulic acid
, caffeic acid
and sinapinic acid
as the matrix. The availability of small and relatively inexpensive nitrogen lasers operating at 337 nm wavelength and the first commercial instruments introduced in the early 1990s brought MALDI to an increasing number of researchers. Today, mostly organic matrices are used for MALDI mass spectrometry.
Anode ray
Anode rays are beams of positive ions that are created by certain types of gas discharge tubes. They were first observed in Crookes tubes during experiments by the German scientist Eugen Goldstein, in 1886. Later work on anode rays by Wilhelm Wien and J. J...
and cathode ray
Cathode ray
Cathode rays are streams of electrons observed in vacuum tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, the glass opposite of the negative electrode is observed to glow, due to electrons emitted from and travelling perpendicular to the cathode Cathode...
s, which turned out to be positive ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s and electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s. Improved capabilities in the separation of these positive ions enabled the discovery of stable isotope
Stable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
s of the elements. The first such discovery was with the atom neon
Neon
Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or...
, which was shown by mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
to have at least two stable isotopes: neon-20 with 10 proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s and 10 neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s and neon-22 with 10 protons and 12 neutrons. Mass spectrometers were used in the Manhattan Project
Manhattan Project
The Manhattan Project was a research and development program, led by the United States with participation from the United Kingdom and Canada, that produced the first atomic bomb during World War II. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army...
for the separation of isotopes of uranium necessary to create the atomic bomb.
Prout's Hypothesis
Prout's hypothesisProut's hypothesis
Prout's hypothesis was an early 19th century attempt to explain the existence of the various chemical elements through a hypothesis regarding the internal structure of the atom...
was an early 19th century attempt to explain the properties of the chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
s using the internal structure of the atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
. In 1815, the English chemist William Prout
William Prout
William Prout FRS was an English chemist, physician, and natural theologian. He is remembered today mainly for what is called Prout's hypothesis.-Biography:...
observed that the atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
s that had been measured were integer
Integer
The integers are formed by the natural numbers together with the negatives of the non-zero natural numbers .They are known as Positive and Negative Integers respectively...
multiples of the atomic weight of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. Prout's hypothesis remained influential in chemistry throughout the 1820s. However, more careful measurements of the atomic weights, such as those compiled by Jöns Jakob Berzelius
Jöns Jakob Berzelius
Jöns Jacob Berzelius was a Swedish chemist. He worked out the modern technique of chemical formula notation, and is together with John Dalton, Antoine Lavoisier, and Robert Boyle considered a father of modern chemistry...
in 1828 or Edward Turner in 1832, appeared to disprove it. In particular the atomic weight of chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, which is 35.45 times that of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, could not at the time be explained in terms of Prout's hypothesis. It would take the better part of a century for this problem to be resolved.
Canal rays
In the mid-nineteenth century, Julius PlückerJulius Plücker
Julius Plücker was a German mathematician and physicist. He made fundamental contributions to the field of analytical geometry and was a pioneer in the investigations of cathode rays that led eventually to the discovery of the electron. He also vastly extended the study of Lamé curves.- Early...
investigated the light emitted in discharge tubes and the influence of magnetic fields on the glow. Later, in 1869, Johann Wilhelm Hittorf
Johann Wilhelm Hittorf
Johann Wilhelm Hittorf was a German physicist who was born in Bonn and died in Münster, Germany.Hittorf was the first to compute the electricity-carrying capacity of charged atoms and molecules , an important factor in understanding electrochemical reactions...
studied discharge tubes with energy rays extending from a negative electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
, the cathode. These rays produced a fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
when they hit a tube's glass walls, and when interrupted by a solid object they cast a shadow.
Canal rays, also called Anode rays, were observed by Eugen Goldstein
Eugen Goldstein
Eugen Goldstein was a German physicist. He was an early investigator of discharge tubes, the discoverer of anode rays, and is sometimes credited with the discovery of the proton.- Life :...
, in 1886. Goldstein used a gas discharge tube
Gas filled tube
A gas-filled tube, also known as a discharge tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Although the envelope is typically glass, power tubes often use ceramics, and military tubes often use glass-lined metal...
which had a perforated cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
. The rays are produced in the holes (canals) in the cathode and travels in a direction opposite to the "cathode ray
Cathode ray
Cathode rays are streams of electrons observed in vacuum tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, the glass opposite of the negative electrode is observed to glow, due to electrons emitted from and travelling perpendicular to the cathode Cathode...
s," which are streams of electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s. Goldstein called these positive rays "Kanalstrahlen" - canal rays. In 1907 a study of how this "ray" was deflected in a magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
, revealed that the particles
Particle physics
Particle physics is a branch of physics that studies the existence and interactions of particles that are the constituents of what is usually referred to as matter or radiation. In current understanding, particles are excitations of quantum fields and interact following their dynamics...
making up the ray were not all the same mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
Discovery of isotopes

J. J. Thomson
Sir Joseph John "J. J." Thomson, OM, FRS was a British physicist and Nobel laureate. He is credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer...
channeled a stream of ionized neon through a magnetic and an electric field and measured its deflection by placing a photographic plate in its path. Thomson observed two patches of light on the photographic plate (see image on right), which suggested two different parabolas of deflection. Thomson concluded that the neon gas was composed of atoms of two different atomic masses (neon-20 and neon-22).
Thomson's student Francis William Aston
Francis William Aston
Francis William Aston was a British chemist and physicist who won the 1922 Nobel Prize in Chemistry for his discovery, by means of his mass spectrograph, of isotopes, in a large number of non-radioactive elements, and for his enunciation of the whole-number rule...
continued the research at the Trinity College, building the first full functional mass spectrometer in 1919. He was able to identify isotopes of chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
(35 and 37), bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
(79 and 81), and krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other...
(78, 80, 82, 83, 84 and 86), proving that these natural occurring elements are composed of a combination of isotopes. The use of electromagnetic focusing in mass spectrograph
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
which rapidly allowed him to identify no fewer than 212 of the 287 naturally occurring isotopes. In 1921 F. W. Aston became a fellow of the famous Royal Society
Royal Society
The Royal Society of London for Improving Natural Knowledge, known simply as the Royal Society, is a learned society for science, and is possibly the oldest such society in existence. Founded in November 1660, it was granted a Royal Charter by King Charles II as the "Royal Society of London"...
.
His work on isotopes also led to his formulation of the Whole Number Rule
Whole number rule
The whole number rule states that the masses of the elements are whole number multiples of the mass of the hydrogen atom. The rule can be formulated from Prout's hypothesis put forth in 1815. In 1920, Francis W...
which states that "the mass of the oxygen isotope being defined [as 16], all the other isotopes have masses that are very nearly whole numbers," a rule that was used extensively in the development of nuclear energy
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
. The exact mass of many isotopes was measured leading to the result that hydrogen has a 1% higher mass than expected by the average mass of the other elements. Aston speculated about the subatomic energy and the use of it in 1936.
In 1918, Arthur Jeffrey Dempster
Arthur Jeffrey Dempster
Arthur Jeffrey Dempster was a Canadian-American physicist best known for his work in mass spectrometry and his discovery of the uranium isotope 235U.-Biography:...
developed the first modern mass spectrometer
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
, which was over 100 times more accurate than previous versions, and established the basic theory and design of mass spectrometers that is still used to this day. Dempster's research over his career centered around the mass spectrometer and its applications, leading in 1935 to his discovery of the uranium isotope 235U. This isotope's ability to cause a rapidly expanding fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
nuclear chain reaction
Nuclear chain reaction
A nuclear chain reaction occurs when one nuclear reaction causes an average of one or more nuclear reactions, thus leading to a self-propagating number of these reactions. The specific nuclear reaction may be the fission of heavy isotopes or the fusion of light isotopes...
allowed the development of the atom bomb and nuclear power
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
.
In 1932, Kenneth Bainbridge
Kenneth Bainbridge
Kenneth Tompkins Bainbridge was an American physicist at Harvard University who did work on cyclotron research. His precise measurements of mass differences between nuclear isotopes allowed him to confirm Albert Einstein's mass-energy equivalence concept. He was the Director of the Trinity test of...
developed a mass spectrometer with a resolving power of 600 and a relative precision of one part in 10,000. He used this instrument to verify the equivalence of mass and energy
Mass-energy equivalence
In physics, mass–energy equivalence is the concept that the mass of a body is a measure of its energy content. In this concept, mass is a property of all energy, and energy is a property of all mass, and the two properties are connected by a constant...
, E = mc2.
Manhattan Project
A CalutronCalutron
A calutron is a mass spectrometer used for separating the isotopes of uranium. It was developed by Ernest O. Lawrence during the Manhattan Project and was similar to the cyclotron invented by Lawrence. Its name is a concatenation of Cal. U.-tron, in tribute to the University of California,...
is a sector mass spectrometer
Sector instrument
A sector instrument is a general term for a class of mass spectrometer that uses a static electric or magnetic sector or some combination of the two as a mass analyzer. A popular combination of these sectors has been the BEB...
that was used for separating the isotopes of uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
developed by Ernest O. Lawrence during the Manhattan Project
Manhattan Project
The Manhattan Project was a research and development program, led by the United States with participation from the United Kingdom and Canada, that produced the first atomic bomb during World War II. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army...
and was similar to the Cyclotron
Cyclotron
In technology, a cyclotron is a type of particle accelerator. In physics, the cyclotron frequency or gyrofrequency is the frequency of a charged particle moving perpendicularly to the direction of a uniform magnetic field, i.e. a magnetic field of constant magnitude and direction...
invented by Lawrence. Its name is a concatenation
Concatenation
In computer programming, string concatenation is the operation of joining two character strings end-to-end. For example, the strings "snow" and "ball" may be concatenated to give "snowball"...
of Cal. U.-tron, in tribute to the University of California
University of California
The University of California is a public university system in the U.S. state of California. Under the California Master Plan for Higher Education, the University of California is a part of the state's three-tier public higher education system, which also includes the California State University...
, Lawrence's institution and the contractor of the Los Alamos
Los Alamos National Laboratory
Los Alamos National Laboratory is a United States Department of Energy national laboratory, managed and operated by Los Alamos National Security , located in Los Alamos, New Mexico...
laboratory. They were implemented for industrial scale uranium enrichment at the Oak Ridge, Tennessee
Oak Ridge, Tennessee
Oak Ridge is a city in Anderson and Roane counties in the eastern part of the U.S. state of Tennessee, about west of Knoxville. Oak Ridge's population was 27,387 at the 2000 census...
Y-12 plant
Y-12 National Security Complex
The Y-12 National Security Complex is a United States Department of Energy National Nuclear Security Administration facility located in Oak Ridge, Tennessee, near the Oak Ridge National Laboratory...
established during the war and provided much of the uranium used for the "Little Boy
Little Boy
"Little Boy" was the codename of the atomic bomb dropped on Hiroshima on August 6, 1945 by the Boeing B-29 Superfortress Enola Gay, piloted by Colonel Paul Tibbets of the 393rd Bombardment Squadron, Heavy, of the United States Army Air Forces. It was the first atomic bomb to be used as a weapon...
" nuclear weapon
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
, which was dropped onto Hiroshima
Hiroshima
is the capital of Hiroshima Prefecture, and the largest city in the Chūgoku region of western Honshu, the largest island of Japan. It became best known as the first city in history to be destroyed by a nuclear weapon when the United States Army Air Forces dropped an atomic bomb on it at 8:15 A.M...
in 1945.
Development of gas chromatography-mass spectrometry
The use of a mass spectrometer as the detector in gas chromatography was developed during the 1950s by Roland Gohlke and Fred McLafferty. The development of affordable and miniaturized computers has helped in the simplification of the use of this instrument, as well as allowed great improvements in the amount of time it takes to analyse a sample.Fourier transform mass spectrometry
Fourier transform ion cyclotron resonanceFourier transform ion cyclotron resonance
Fourier transform ion cyclotron resonance mass spectrometry, also known as Fourier transform mass spectrometry, is a type of mass analyzer for determining the mass-to-charge ratio of ions based on the cyclotron frequency of the ions in a fixed magnetic field...
mass spectrometry was developed by Alan G. Marshall
Alan G. Marshall
Alan G. Marshall is an American analytical chemist who has devoted his scientific career to developing a scientific technique known as Fourier transform ion cyclotron resonance mass spectrometry, which he co-invented. He was born in Bluffton, Ohio, in 1944, and earned his Bachelor's in Chemistry...
and Melvin B. Comisarow at the University of British Columbia
University of British Columbia
The University of British Columbia is a public research university. UBC’s two main campuses are situated in Vancouver and in Kelowna in the Okanagan Valley...
in 1974. The inspiration was earlier developments in conventional ICR and Fourier Transform Nuclear Magnetic Resonance (FT-NMR) spectroscopy.
Soft ionization methods
Field desorption ionization was first reported by Beckey in 1969. In field ionization, a high-potential electric field is applied to an emitter with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have been grown. This produces a very high electric field in which electron tunneling can result in ionization of gaseous analyte molecules. FI produces mass spectra with little or no fragmentation, dominated by molecular radical cations M+. and occasionally protonated molecules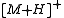 .
.Chemical ionization was developed in the 1960s. Ionization of sample (analyte) is achieved by interaction of its molecules with reagent ions. The analyte is ionized by ion-molecule reactions during collisions in the source. The process may involve transfer of an electron, a proton or other charged species between the reactants. This is a less energetic procedure than electron ionization
Electron ionization
Electron ionization is an ionization method in which energetic electrons interact with gas phase atoms or molecules to produce ions...
and the ions produced are, for example, protonated molecules: [M + H]+. These ions are often relatively stable, tending not to fragment as readily as ions produced by electron ionization
Electron ionization
Electron ionization is an ionization method in which energetic electrons interact with gas phase atoms or molecules to produce ions...
.
Matrix-assisted laser desorption/ionization
Matrix-assisted laser desorption/ionization
Matrix-assisted laser desorption/ionization is a soft ionization technique used in mass spectrometry, allowing the analysis of biomolecules and large organic molecules , which tend to be fragile and fragment when ionized by more conventional ionization methods...
(MALDI) is a soft ionization
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
technique used in mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
, allowing the analysis of biomolecule
Biomolecule
A biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
s (biopolymer
Biopolymer
Biopolymers are polymers produced by living organisms. Since they are polymers, Biopolymers contain monomeric units that are covalently bonded to form larger structures. There are three main classes of biopolymers based on the differing monomeric units used and the structure of the biopolymer formed...
s such as proteins, peptides and sugars) and large organic
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
molecules (such as polymers, dendrimers and other macromolecules), which tend to be fragile and fragment when ionized by more conventional ionization methods. It is most similar in character to electrospray ionization
Electrospray ionization
Electrospray ionization is a technique used in mass spectrometry to produce ions. It is especially useful in producing ions from macromolecules because it overcomes the propensity of these molecules to fragment when ionized...
both in relative softness and the ions produced (although it causes much fewer multiply charged ions). The term was first used in 1985 by Franz Hillenkamp
Franz Hillenkamp
Franz Hillenkamp is a German mass spectrometry scientist developer with Michael Karas of the matrix-assisted laser desorption/ionization technique.-Awards:...
, Michael Karas
Michael Karas
Michael Karas is a German physical chemistry scientist and Professor, known for his researches on matrix-assisted laser desorption/ionization , a technique in mass spectrometry....
and their colleagues. These researchers found that the amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
could be ionized more easily if it was mixed with the amino acid tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
and irradiated with a pulsed 266 nm laser. The tryptophan was absorbing the laser energy and helping to ionize the non-absorbing alanine. Peptides up to the 2843 Da peptide melittin
Melittin
Melittin is the principal active component of apitoxin and is a powerful stimulator of phospholipase A2. Melittin is a peptide consisting of 26 amino acids with the sequence GIGAVLKVLTTGLPALISWIKRKRQQ.-Biological effects:...
could be ionized when mixed with this kind of “matrix”.
The breakthrough for large molecule laser desorption ionization came in 1987 when Koichi Tanaka
Koichi Tanaka
is a Japanese scientist who shared the Nobel Prize in Chemistry in 2002 for developing a novel method for mass spectrometric analyses of biological macromolecules with John Bennett Fenn and Kurt Wuthrich ....
of Shimadzu Corp. and his co-workers used what they called the “ultra fine metal plus liquid matrix method” that combined 30 nm cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
particles in glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
with a 337 nm nitrogen laser
Nitrogen laser
A nitrogen laser is a gas laser operating in the ultraviolet range using molecular nitrogen as its gain medium, pumped by an electrical discharge....
for ionization. Using this laser and matrix combination, Tanaka was able to ionize biomolecules as large as the 34,472 Da protein carboxypeptidase-A. Tanaka received one-quarter of the 2002 Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
for demonstrating that, with the proper combination of laser wavelength and matrix, a protein can be ionized. Karas and Hillenkamp were subsequently able to ionize the 67 kDa protein albumin using a nicotinic acid matrix and a 266 nm laser. Further improvements were realized through the use of a 355 nm laser and the cinnamic acid
Cinnamic acid
Cinnamic acid is a white crystalline organic acid, which is slightly soluble in water.It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter and is the best indication of its environmental history and post-extraction conditions...
derivatives ferulic acid
Ferulic acid
Ferulic acid is a hydroxycinnamic acid, a type of organic compound. It is an abundant phenolic phytochemical found in plant cell wall components such as arabinoxylans as covalent side chains. It is related to trans-cinnamic acid. As a component of lignin, ferulic acid is a precursor in the...
, caffeic acid
Caffeic acid
Caffeic acid is a hydroxycinnamic acid, a naturally occurring organic compound. This yellow solid consists of both phenolic and acrylic functional groups...
and sinapinic acid
Sinapinic acid
Sinapinic acid, or sinapic acid , is a small naturally occurring hydroxycinnamic acid. It is a member of the phenylpropanoid family. It is a commonly used matrix in MALDI mass spectrometry. It is a useful matrix for a wide variety of peptides and proteins...
as the matrix. The availability of small and relatively inexpensive nitrogen lasers operating at 337 nm wavelength and the first commercial instruments introduced in the early 1990s brought MALDI to an increasing number of researchers. Today, mostly organic matrices are used for MALDI mass spectrometry.
19th century
- 1886
- Eugen GoldsteinEugen GoldsteinEugen Goldstein was a German physicist. He was an early investigator of discharge tubes, the discoverer of anode rays, and is sometimes credited with the discovery of the proton.- Life :...
observes canal rays.
- Eugen Goldstein
- 1898
- Wilhelm WienWilhelm WienWilhelm Carl Werner Otto Fritz Franz Wien was a German physicist who, in 1893, used theories about heat and electromagnetism to deduce Wien's displacement law, which calculates the emission of a blackbody at any temperature from the emission at any one reference temperature.He also formulated an...
demonstrates that canal rays can be deflected using strong electric and magnetic fields. He shows that the mass-to-charge ratioMass-to-charge ratioThe mass-to-charge ratio ratio is a physical quantity that is widely used in the electrodynamics of charged particles, e.g. in electron optics and ion optics. It appears in the scientific fields of lithography, electron microscopy, cathode ray tubes, accelerator physics, nuclear physics, Auger...
of the particles have opposite polarity and is much larger compared to the electron. He also realizes that the particle mass is similar to the one of hydrogen particle.
- Wilhelm Wien
- 1898
- J. J. ThomsonJ. J. ThomsonSir Joseph John "J. J." Thomson, OM, FRS was a British physicist and Nobel laureate. He is credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer...
measures the mass-to-charge ratioMass-to-charge ratioThe mass-to-charge ratio ratio is a physical quantity that is widely used in the electrodynamics of charged particles, e.g. in electron optics and ion optics. It appears in the scientific fields of lithography, electron microscopy, cathode ray tubes, accelerator physics, nuclear physics, Auger...
of electrons.
- J. J. Thomson
20th century
- 1901
- Walter KaufmannWalter Kaufmann (physicist)Walter Kaufmann was a German physicist. He is most well known for his first experimental proof of the velocity dependence of mass, which was an important contribution to the development of modern physics, including special relativity.-Life:In 1890/91 he studied mechanical engineering at the...
uses a mass spectrometer to measure the relativistic mass increase of electrons.
- Walter Kaufmann
- 1905
- J. J. ThomsonJ. J. ThomsonSir Joseph John "J. J." Thomson, OM, FRS was a British physicist and Nobel laureate. He is credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer...
begins his study of positive rays.
- J. J. Thomson
- 1906
- Thomson is awarded the Nobel Prize in Physics "in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases"
- 1913
- Thomson is able to separate particles of different mass-to-charge ratioMass-to-charge ratioThe mass-to-charge ratio ratio is a physical quantity that is widely used in the electrodynamics of charged particles, e.g. in electron optics and ion optics. It appears in the scientific fields of lithography, electron microscopy, cathode ray tubes, accelerator physics, nuclear physics, Auger...
s. He separates the 20Ne and the 22Ne isotopes, and he correctly identifies the m/z = 11 signal as a doubly charged 22Ne particle.
- Thomson is able to separate particles of different mass-to-charge ratio
- 1919
- Francis Aston constructs the first velocity focusing mass spectrograph with mass resolving power of 130.
- 1922
- Aston is awarded the Nobel Prize in chemistry "for his discovery, by means of his mass spectrograph, of isotopes, in a large number of non-radioactive elements, and for his enunciation of the whole-number rule."
- 1931
- Ernest O. Lawrence invents the cyclotronCyclotronIn technology, a cyclotron is a type of particle accelerator. In physics, the cyclotron frequency or gyrofrequency is the frequency of a charged particle moving perpendicularly to the direction of a uniform magnetic field, i.e. a magnetic field of constant magnitude and direction...
.
- Ernest O. Lawrence invents the cyclotron
- 1934
- Josef MattauchJosef MattauchJosef Mattauch was a German physicist known for his work in the investigation of the isotopic abundances by mass spectrometry. He developed the Mattauch isobar rule in 1934.-Mattauch-Herzog geometry mass spectrometer:...
and Richard Herzog develop the double-focusing mass spectrograph.
- Josef Mattauch
- 1936
- Arthur J. Dempster develops the spark ionizationSpark ionizationSpark ionization is a method used to produce gas phase ions from a solid sample. The prepared solid sample is vaporized and partially ionized by an intermittent discharge or spark. This technique is primarily used in the field of mass spectrometry...
source.
- Arthur J. Dempster develops the spark ionization
- 1937
- Aston constructs a mass spectrograph with resolving power of 2000.
- 1939
- Lawrence receives the Nobel Prize in Physics for the cyclotron.
- 1942
- Lawrence develops the CalutronCalutronA calutron is a mass spectrometer used for separating the isotopes of uranium. It was developed by Ernest O. Lawrence during the Manhattan Project and was similar to the cyclotron invented by Lawrence. Its name is a concatenation of Cal. U.-tron, in tribute to the University of California,...
for uraniumUraniumUranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
isotope separationIsotope separationIsotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes, for example separating natural uranium into enriched uranium and depleted uranium. This is a crucial process in the manufacture of uranium fuel for nuclear power stations, and is...
.
- Lawrence develops the Calutron
- 1943
- Westinghouse markets it's Mass Spectrometer and proclaims it to be "A New Electronic Method for fast, accurate gas analysis"
- 1946
- William Stephens presents the concept of a time-of-flightTime-of-flight mass spectrometryTime-of-flight mass spectrometry is a method of mass spectrometry in which an ion's mass-to-charge ratio is determined via a time measurement. Ions are accelerated by an electric field of known strength. This acceleration results in an ion having the same kinetic energy as any other ion that has...
mass spectrometer.
- William Stephens presents the concept of a time-of-flight
- 1956
- Fred McLafferty proposes a hydrogen transfer reaction that will come to be known as the McLafferty rearrangementMcLafferty rearrangementThe McLafferty rearrangement is a reaction observed in mass spectrometry. It is sometimes found that a molecule containing a keto-group undergoes β-cleavage, with the gain of the γ-hydrogen atom...
.
- Fred McLafferty proposes a hydrogen transfer reaction that will come to be known as the McLafferty rearrangement
- 1959
- Researchers at Dow Chemical interface a gas chromatograph to a mass spectrometer.
- 1966
- F. H. Field and M. S. B. Munson develop chemical ionizationChemical ionizationChemical ionization is an ionization technique used in mass spectrometry. Chemical ionization is a lower energy process than electron ionization. The lower energy yields less fragmentation, and usually a simpler spectrum...
.
- F. H. Field and M. S. B. Munson develop chemical ionization
- 1968
- Malcolm DoleMalcolm DoleMalcolm Dole was an American chemist known for the Dole Effect in which he proved that the atomic weight of oxygen in air is greater than that of oxygen in water and for his work on electrospray ionization, polymer chemistry, and electrochemistry.- Selected writings : ; -External links:*...
develops electrospray ionization.
- Malcolm Dole
- 1969
- H. D. Beckey develops field desorptionField desorptionField desorption /field ionization refers to an ion source for mass spectrometry first reported by Beckey in 1969. In field ionization, a high-potential electric field is applied to an emitter with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers"...
.
- H. D. Beckey develops field desorption
- 1974
- Comisarow and Marshall develop Fourier Transform Ion Cyclotron ResonanceFourier transform ion cyclotron resonanceFourier transform ion cyclotron resonance mass spectrometry, also known as Fourier transform mass spectrometry, is a type of mass analyzer for determining the mass-to-charge ratio of ions based on the cyclotron frequency of the ions in a fixed magnetic field...
Mass Spectrometry
- Comisarow and Marshall develop Fourier Transform Ion Cyclotron Resonance
- 1976
- Ronald MacFarlane and co-workers develop plasma desorption mass spectrometryPlasma desorption mass spectrometryPlasma desorption ionization mass spectrometry is a mass spectrometry technique in which ionization of material in a solid sample by bombarding it with ionic or neutral atoms formed as a result of the nuclear fission of a suitable nuclide, typically the Californium isotope 252Cf.-See also:* Fast...
.
- Ronald MacFarlane and co-workers develop plasma desorption mass spectrometry
- 1984
- John Bennett Fenn and co-workers use electrosprayElectrosprayThe name electrospray is used for a device that employs electricity to disperse a liquid or for the fine aerosol resulted in this process. The method is sometimes improperly called electrohydrodynamic atomization. High voltage is applied to a liquid supplied through an emitter...
to ionize biomolecules.
- John Bennett Fenn and co-workers use electrospray
- 1985
- Franz Hillenkamp, Michael Karas and co-workers describe and coin the term matrix-assisted laser desorption ionization (MALDI).
- 1987
- Koichi TanakaKoichi Tanakais a Japanese scientist who shared the Nobel Prize in Chemistry in 2002 for developing a novel method for mass spectrometric analyses of biological macromolecules with John Bennett Fenn and Kurt Wuthrich ....
uses the “ultra fine metal plus liquid matrix method” to ionize intact proteins.
- Koichi Tanaka
- 1989
- Wolfgang PaulWolfgang PaulWolfgang Paul was a German physicist, who co-developed the non-magnetic quadrupole mass filter which laid the foundation for what we now call an ion trap...
receives the Nobel Prize in Physics "for the development of the ion trap technique"
- Wolfgang Paul
- 1999
- Alexander Makarov presents the OrbitrapOrbitrapAn orbitrap is a type of mass spectrometer invented by Alexander Makarov. It consists of an outer barrel-like electrode and a coaxial inner spindle-like electrode that form an electrostatic field with quadro-logarithmic potential distribution....
mass spectrometer
- Alexander Makarov presents the Orbitrap
21st century
- 2002
- John Bennett Fenn and Koichi TanakaKoichi Tanakais a Japanese scientist who shared the Nobel Prize in Chemistry in 2002 for developing a novel method for mass spectrometric analyses of biological macromolecules with John Bennett Fenn and Kurt Wuthrich ....
are awarded one-quarter of the Nobel Prize in chemistry each "for the development of soft desorption ionisation methods ... for mass spectrometric analyses of biological macromolecules."
- John Bennett Fenn and Koichi Tanaka

