Insertion reaction
Encyclopedia
An insertion reaction is a chemical reaction
where one chemical entity (a molecule
or molecular fragment) interposes itself into an existing bond
of typically a second chemical entity e.g.:
The term only refers to the result of the reaction and does not suggest a mechanism. Insertion reactions are observed in organic
, inorganic
, and organometallic
chemistry. In cases where a metal-ligand
bond in a coordination complex is involved, these reactions are typically organometallic in nature and involve a bond between a transition metal
and a carbon
or hydrogen
. It is usually reserved for the case where the coordination number
and oxidation state
of the metal remain unchanged. When these reactions are reversible, the removal of the small molecule from the metal-ligand bond is called extrusion or elimination.
There are two common insertion geometries— 1,1 and 1,2 (pictured above). Additionally, the inserting molecule can act either as a nucleophile
or as an electrophile
to the metal complex. These behaviors will be discussed in more detail for CO
, nucleophilic behavior, and SO2
, electrophilic behavior.
s like the Kowalski ester homologation
provide simple examples of insertion process in organic synthesis. In the Arndt-Eistert reaction, a methylene
unit is inserted into the carboxyl-carbon bond of carboxylic acid
to form the next acid in the homologous series
. Organic Syntheses
provides the example of t-BOC protected
(S)-phenylalanine
(2-amino-3-phenylpropanoic acid) being reacted sequentially with triethylamine
, ethyl chloroformate
, and diazomethane
to produce the α-diazoketone
, which is then reacted with silver trifluoroacetate / triethylamine in aqueous solution to generate the t-BOC protected form of (S)-3-amino-4-phenylbutanoic acid.
Mechanistically, the α-diazoketone undergoes a Wolff rearrangement
to form a ketene
in a 1,2-rearrangement
. Consequently, the methylene group α- to the carboxyl group in the product is the methylene group from the diazomethane reagant. The 1,2-rearrangement has been shown to conserve the stereochemistry of the chiral centre as the product formed from t-BOC protected (S)-phenylalanine retains the (S) stereochemistry with a reported enantiomeric excess
of at least 99%.
A related transformation is the Nierenstein reaction
in which a diazomethane methylene group is inserted into the carbon-chlorine bond of an acid chloride to generate an α-chloromethyl ketone. An example, published in 1924, illustrates the reaction in a substituted benzoyl chloride
system:
Perhaps surprisingly, α-bromoacetophenone
is the minor product when this reaction is carried out with benzoyl bromide
, a dimeric dioxane being the major product. Organic azides also provide an example of an insertion reaction in organic synthesis and, like the above examples, the transformations proceed with loss of nitrogen gas
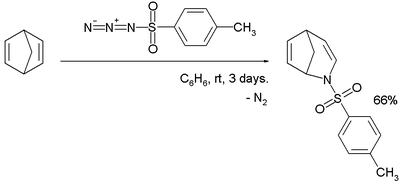
. When tosyl azide
reacts with norbornadiene
, a ring expansion reaction takes place in which a nitrogen atom is inserted into a carbon-carbon bond α- to the bridge head:
The Beckmann rearrangement
is another example of a ring expanding reaction in which a heteroatom is inserted into a carbon-carbon bond. The most important application of this reaction is the conversion of cyclohexanone
to its oxime, which is then rearranged under acidic conditions to provide ε-caprolactam
, the feedstock for the manufacture of Nylon 6
. Annual production of caprolactam exceeds 2 billion kilograms.
Carbene
s undergo both intermolecular and intramolecular
insertion reactions. Cyclopentene
moieties can be generated from sufficiently long-chain ketone
s by reaction with trimethylsilyldiazomethane
, (CH3)3Si–CHN2:
Here, the carbene intermediate inserts into a carbon-hydrogen bond to form the carbon-carbon bond needed to close the cyclopentene ring. Carbene insertions into carbon-hydrogen bonds
can also occur intermolecularly:
Carbenoid
s are reactive intermediate
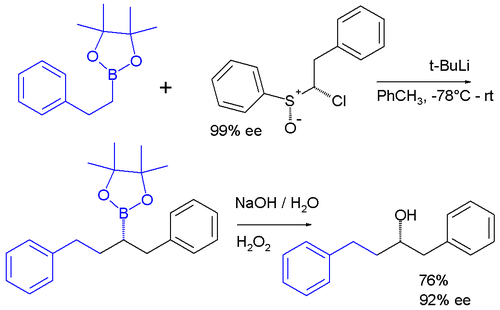
s that behave similarly to carbenes. One example is the chloroalkyllithium carbenoid reagent prepared in situ from a sulfoxide
and t-BuLi which inserts into the carbon-boron bond of a pinacol boronic ester:
 Such reactions are subject to the usual parameters that affect other reactions in coordination chemistry, but steric effects
Such reactions are subject to the usual parameters that affect other reactions in coordination chemistry, but steric effects
are especially important in determining the stereochemistry and regiochemistry of the reactions. The reverse reaction, the de-insertion of CO and alkenes, are of fundamental significance in many catalytic cycles as well.
Widely employed applications of migratory insertion of carbonyl groups are hydroformylation
and the carbonylative production of acetic acid. The former converts alkenes, hydrogen, and carbon monoxide into aldehydes. The production of acetic acid by carbonylation proceeds via two similar industrial processes. More traditional is the rhodium-based Monsanto acetic acid process
, but this process has been superseded by the iridium-based Cativa process
. By 2002, worldwide annual production of acetic acid stood at 6 million tons, of which approximately 60% is produced by the Cativa process.
The Cativa process catalytic cycle
, shown above, includes both insertion and de-insertion steps. The oxidative addition reaction of methyl iodide with (1) involves the formal insertion of the iridium(I) centre into the carbon-iodine bond, whereas step (3) to (4) is an example of migratory insertion of carbon monoxide into the iridium-carbon bond. The active catalyst species is regenerated by the reductive elimination of acetyl iodide
from (4), a de-insertion reaction.
which produces detergent
precursors. the olefin can be coordinated to the metal before insertion. Depending on the ligand density of the metal, ligand dissociation may be necessary to provide a coordination site for the olefin.
, carbon dioxide
, and nitric oxide. These reactions have limited practical significance, but are of historic interest. With transition metal alkyls, these oxides behave as electrophiles and insert into the bond between metals and their relatively nucleophilic alkyl ligands. As discussed in the article on Metal sulfur dioxide complex
es, the insertion of SO2 has been examined in particular detail.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
where one chemical entity (a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
or molecular fragment) interposes itself into an existing bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
of typically a second chemical entity e.g.:
- A + B–C → B–A–C
The term only refers to the result of the reaction and does not suggest a mechanism. Insertion reactions are observed in organic
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, inorganic
Inorganic chemistry
Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
, and organometallic
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
chemistry. In cases where a metal-ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
bond in a coordination complex is involved, these reactions are typically organometallic in nature and involve a bond between a transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
and a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
or hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. It is usually reserved for the case where the coordination number
Coordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
and oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of the metal remain unchanged. When these reactions are reversible, the removal of the small molecule from the metal-ligand bond is called extrusion or elimination.
There are two common insertion geometries— 1,1 and 1,2 (pictured above). Additionally, the inserting molecule can act either as a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
or as an electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
to the metal complex. These behaviors will be discussed in more detail for CO
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, nucleophilic behavior, and SO2
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
, electrophilic behavior.
Organic chemistry
Homologation reactionHomologation reaction
A homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A Homologous series is a group of compounds that differ by a constant unit, generally a group. The reactants undergo a homologation when the...
s like the Kowalski ester homologation
Kowalski ester homologation
The Kowalski ester homologation is a chemical reaction for the homologation of esters.This reaction was designed as a safer alternative to the Arndt-Eistert synthesis. The Kowalski reaction is named after its inventor, Conrad J. Kowalski....
provide simple examples of insertion process in organic synthesis. In the Arndt-Eistert reaction, a methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
unit is inserted into the carboxyl-carbon bond of carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
to form the next acid in the homologous series
Homologous series
In chemistry, a homologous series is a series of compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group, and showing a gradation in physical properties as a result of increase in molecular size and mass...
. Organic Syntheses
Organic Syntheses
Organic Syntheses is a scientific journal that since 1921 has provided the chemistry community with annual collections of detailed and checked procedures for the organic synthesis of organic compounds. The journal is peer reviewed...
provides the example of t-BOC protected
Di-tert-butyl dicarbonate
Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. This carbonate ester reacts with amines to give N-tert-butoxycarbonyl or so-called t-BOC derivatives. These derivatives do not behave as amines, which allows certain subsequent transformations to occur that would have...
(S)-phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
(2-amino-3-phenylpropanoic acid) being reacted sequentially with triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
, ethyl chloroformate
Ethyl chloroformate
Ethyl chloroformate is the ethyl ester of chloroformic acid. It is a reagent used in organic synthesis for the introduction of the ethyl carbamate protecting group and for the formation of carboxylic anhydrides....
, and diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
to produce the α-diazoketone
Diazo
Diazo refers to a type of organic compound called diazo compound that has two linked nitrogen atoms as a terminal functional group. The general formula is R2C=N2. The simplest example of a diazo compound is diazomethane...
, which is then reacted with silver trifluoroacetate / triethylamine in aqueous solution to generate the t-BOC protected form of (S)-3-amino-4-phenylbutanoic acid.
Mechanistically, the α-diazoketone undergoes a Wolff rearrangement
Wolff rearrangement
The Wolff rearrangement is a rearrangement reaction converting a α-diazo-ketone into a ketene. This reaction was first reported by Ludwig Wolff in 1912....
to form a ketene
Ketene
A ketene is an organic compound of the form R'RC=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R are hydrogen atoms.Ketenes were first studied as a class by Hermann Staudinger.-Formation:...
in a 1,2-rearrangement
1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible...
. Consequently, the methylene group α- to the carboxyl group in the product is the methylene group from the diazomethane reagant. The 1,2-rearrangement has been shown to conserve the stereochemistry of the chiral centre as the product formed from t-BOC protected (S)-phenylalanine retains the (S) stereochemistry with a reported enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
of at least 99%.
A related transformation is the Nierenstein reaction
Nierenstein reaction
The Nierenstein reaction is an organic reaction describing the conversion of an acid chloride into an haloketone with diazomethane. It is an insertion reaction in that the methylene from the diazomethane is inserted into the carbon-chlorine bond of the acid chloride.-Reaction mechanism:Like the...
in which a diazomethane methylene group is inserted into the carbon-chlorine bond of an acid chloride to generate an α-chloromethyl ketone. An example, published in 1924, illustrates the reaction in a substituted benzoyl chloride
Benzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C6H5COCl. It is a colourless, fuming liquid with an irritating odour...
system:
Perhaps surprisingly, α-bromoacetophenone
Phenacyl bromide
Phenacyl bromide is the organic compound with the formula C6H5CCH2Br. This colourless solid is a powerful lachrymator as well as a useful precursor to other organic compounds.It is prepared by bromination of acetophenone:...
is the minor product when this reaction is carried out with benzoyl bromide
Benzoyl
In organic chemistry, benzoyl is the acyl of benzoic acid, with structure C6H5CO-. It should not be confused with benzyl, which is the radical or ion formed from the removal of one of the methyl hydrogens of toluene...
, a dimeric dioxane being the major product. Organic azides also provide an example of an insertion reaction in organic synthesis and, like the above examples, the transformations proceed with loss of nitrogen gas
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
. When tosyl azide
Tosyl azide
Tosyl azide is a reagent used in organic synthesis.-Uses:Tosyl azide is used for the introduction of azide and diazo functional groups. It is also used as a nitrene source and as a substrate for [3+2] cycloaddition reactions.-Preparation:...
reacts with norbornadiene
Norbornadiene
Norbornadiene is an organic compound. This bicyclic hydrocarbon is the most stable diolefin derived from the norbornane and norbornene. Norbornadiene is primarily of interest as a ligand in homogeneous catalysis, but it has been heavily studied due to its high reactivity and distinctive...
, a ring expansion reaction takes place in which a nitrogen atom is inserted into a carbon-carbon bond α- to the bridge head:
The Beckmann rearrangement
Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann , is an acid-catalyzed rearrangement of an oxime to an amide...
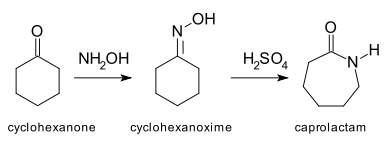
is another example of a ring expanding reaction in which a heteroatom is inserted into a carbon-carbon bond. The most important application of this reaction is the conversion of cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
to its oxime, which is then rearranged under acidic conditions to provide ε-caprolactam
Caprolactam
Caprolactam is an organic compound with the formula 5CNH. This colourless solid is a lactam or a cyclic amide of caproic acid. Approximately 2 billion kilograms are produced annually...
, the feedstock for the manufacture of Nylon 6
Nylon 6
Nylon 6 or polycaprolactam is a polymer developed by Paul Schlack at IG Farben to reproduce the properties of nylon 6,6 without violating the patent on its production. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization. This makes...
. Annual production of caprolactam exceeds 2 billion kilograms.
Carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s undergo both intermolecular and intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
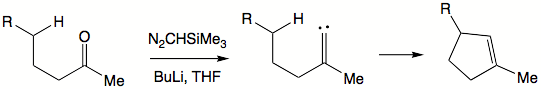
insertion reactions. Cyclopentene
Cyclopentene
Cyclopentene is a chemical compound with the formula 58. It is a colorless liquid with a petrol-like odor. It is one of the cycloalkenes.Cyclopentene is produced industrially in large amounts...
moieties can be generated from sufficiently long-chain ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s by reaction with trimethylsilyldiazomethane
Trimethylsilyldiazomethane
Trimethylsilyldiazomethane, 3SiCHN2, is a diazo compound widely used as a less-explosive replacement for diazomethane.-Preparation:Trimethylsilyldiazomethane may be prepared from reacting methylmagnesium chloride with diphenyl phosphorazidate.-Uses:Trimethylsilyldiazomethane is a commercially...
, (CH3)3Si–CHN2:
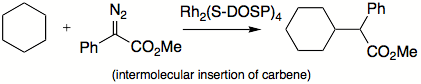
Here, the carbene intermediate inserts into a carbon-hydrogen bond to form the carbon-carbon bond needed to close the cyclopentene ring. Carbene insertions into carbon-hydrogen bonds
Carbene C-H insertion
Carbene C−H insertion in organic chemistry concerns the insertion reaction of a carbene into a carbon–hydrogen bond. This organic reaction is of some importance in the synthesis of new organic compounds . Simple carbenes such as methylene and dichlorocarbene are not regioselective towards...
can also occur intermolecularly:
Carbenoid
Carbenoid
In chemistry a carbenoid is a reactive intermediate that shares reaction characteristics with a carbene. In the Simmons-Smith reaction the carbenoid intermediate is a zinc / iodine complex that takes the form of...
s are reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
s that behave similarly to carbenes. One example is the chloroalkyllithium carbenoid reagent prepared in situ from a sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
and t-BuLi which inserts into the carbon-boron bond of a pinacol boronic ester:
Organometallic chemistry
Many reactions in organometallic chemistry involve insertion of one ligand (L) into a metal-hydride or metal-alkyl/aryl bond. Generally it is the hydride, alkyl, or aryl group that migrates onto L, which is often CO, an alkene, or alkyne.Carbonylations
The insertion of carbon monoxide and alkenes into metal-carbon bonds is a widely exploited reaction with major industrial applications.
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
are especially important in determining the stereochemistry and regiochemistry of the reactions. The reverse reaction, the de-insertion of CO and alkenes, are of fundamental significance in many catalytic cycles as well.
Widely employed applications of migratory insertion of carbonyl groups are hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
and the carbonylative production of acetic acid. The former converts alkenes, hydrogen, and carbon monoxide into aldehydes. The production of acetic acid by carbonylation proceeds via two similar industrial processes. More traditional is the rhodium-based Monsanto acetic acid process
Monsanto process
The Monsanto process is an important method for the manufacture of acetic acid by catalytic carbonylation of methanol. This process operates at a pressure of 30–60 atm and a temperature of 150–200 °C and gives a selectivity greater than 99%. It was developed 1960 by German BASF and...
, but this process has been superseded by the iridium-based Cativa process
Cativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc...
. By 2002, worldwide annual production of acetic acid stood at 6 million tons, of which approximately 60% is produced by the Cativa process.
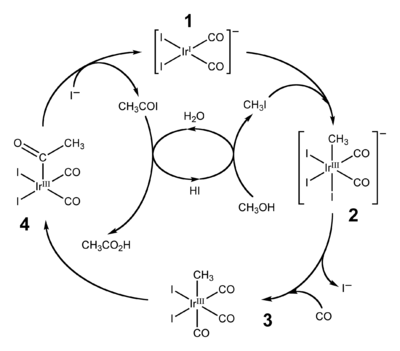
The Cativa process catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
, shown above, includes both insertion and de-insertion steps. The oxidative addition reaction of methyl iodide with (1) involves the formal insertion of the iridium(I) centre into the carbon-iodine bond, whereas step (3) to (4) is an example of migratory insertion of carbon monoxide into the iridium-carbon bond. The active catalyst species is regenerated by the reductive elimination of acetyl iodide
Acetyl iodide
Acetyl iodide is an organoiodine compound wth the formula CH3CI. It is a colourless liquid. It is formally derived from acetic acid. Although far rarer in the laboratory than the related acetyl bromide and acetyl chloride, acetyl iodide is produced, transiently at least, on a far larger scale...
from (4), a de-insertion reaction.
Olefin insertion
The insertion of ethylene and propylene into titanium alkyls is the cornerstone of Ziegler-Natta catalysis, the commercial route of polyethylene and polypropylene. This technology mainly involves heterogeneous catalysts, but it is widely assumed that the principles and observations on homogeneous systems are applicable to the solid-state versions. Related technologies include the Shell Higher Olefin ProcessShell higher olefin process
The Shell higher olefin process is a chemical process for the production of linear alpha olefins via ethylene oligomerization and olefin metathesis invented and exploited by Royal Dutch Shell. The olefin products are converted to fatty aldehydes and then to fatty alcohols, which are precursors...
which produces detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
precursors. the olefin can be coordinated to the metal before insertion. Depending on the ligand density of the metal, ligand dissociation may be necessary to provide a coordination site for the olefin.
Other insertion reactions in coordination chemistry
Many electrophilic oxides insert into metal carbon bonds; these include sulfur dioxideSulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
, carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, and nitric oxide. These reactions have limited practical significance, but are of historic interest. With transition metal alkyls, these oxides behave as electrophiles and insert into the bond between metals and their relatively nucleophilic alkyl ligands. As discussed in the article on Metal sulfur dioxide complex
Metal sulfur dioxide complex
Metal sulfur dioxide complexes are complexes that contain sulfur dioxide, SO2, bonded to a transition metal. Such compounds are common but are mainly of theoretical interest...
es, the insertion of SO2 has been examined in particular detail.