Organocobalt chemistry
Encyclopedia
Organocobalt chemistry is the chemistry
of organometallic compounds containing a carbon
to cobalt
chemical bond
. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12
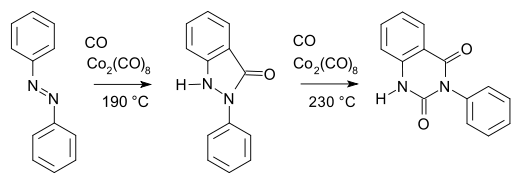
has a cobalt-carbon bond. Many organocobalt compounds are investigated for catalytic properties. An early example of organocobalt chemistry is the carbonylation
of azobenzene
with dicobalt octacarbonyl
as described by Murahashi & Horiie in 1956:
The basic characteristics of organocobalt compounds are:
ed alkynes and cyano compounds. This property is exploited in the use of dicobalt octacarbonyl
as protective group for alkynes. In the Nicholas reaction
an alkyne group is also protected and at the same time the alpha-carbon position is activated for nucleophilic substitution.
(CO insertion) and alkyne trimerization (notably with cyclopentadienylcobalt dicarbonyl
).
reactions and more specifically in hydroformylation
, the formation of aldehydes from an alkene, formaldehyde and hydrogen. An important catalyst in this reaction type is HCo(CO)4 (cobalthydrocarbonyl) at one time used in the industrial production of butyraldehyde from propylene
. In these processes cobalt catalysts are competing with rhodium
catalysts such as HRh(CO)(PPh3)4].
In hydrocarboxylations hydrogen is replaced by water or an alcohol
and the reaction product is a carboxylic acid
or an ester
. An example of this reaction type is the conversion of butadiene to adipic acid
.
Cobalt catalysts (together with iron
) are relevant in the Fischer-Tropsch process
in which synthesis gas is converted to hydrocarbons. The basic reaction sequence is depicted below :
cobalt has an octahedral geometry with a Co-C bond in an axial position. In methylcobalamin the ligand is a methyl group.
s. Cobaltocene
is a 19-electron metallocene, the compound CoCp(C6(Me)6) ha 20 electrons and 21 electrons are counted in Co(C6(Me)6)2 . The Kläui ligand binds metals.
breaks up (by heat or by light) in a carbon free radical and a cobalt(II) radical species. The carbon radical starts polymer chain formation with monomer for instance an alkene as in any ordinary radical polymerization. Cobalt is unusual in that it can reversibly
reform a covalent bond with the carbon radical terminus of the growing chain. This reduces the concentration of radicals to a minimum and also undesirable termination reactions by recombination of two carbon radicals. The cobalt trapping reagent is called a persistent radical and the cobalt-capped polymer chain is said to dormant. CMRP can be regarded as a series of carbometalation
reactions of vinyl monomers. When the monomer possesses protons that can be easily abstracted by the cobalt radical, (catalytic) chain transfer
may occur.
For this reason preferred monomers are acrylic and vinyl esters (e.g. vinyl acetate
), acrylic acid
and acrylonitrile
. The reaction temperature is typically between 0 and 60 °C.
The concept was introduced independently by two groups in 1994.
Much studied cobalt compounds are cobaloximes, cobalt porphyrin
s and Co(acac
)2 derivatives, used in combination with other radical initiators.
Cobalt mediated radical polymerization can also follow different mechanisms, such as catalytic chain transfer
or degenerative transfer:
. In the example of phenylacetylene
a simple catalyst system of cobalt(II) bromide
/ zinc
/ zinc iodide
suffices to obtain 99% chemical yield and 97% regioselectivity
in favor of the ortho substituted reaction product 1.
for trimerization is fairly well understood. A 2007 mechanism consistent with in silico
and experimental data is depicted below for a cobaltocene
catalyst
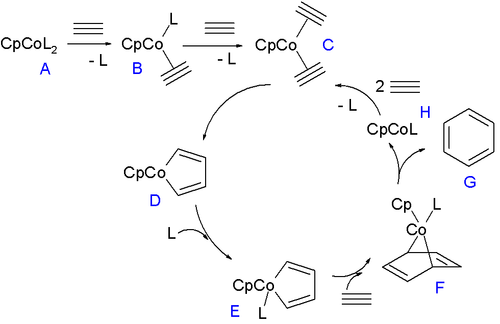 The ligand
The ligand
L in the 18-electron compound
CpCoL2 (A) can be triphenylphosphine
or carbon monoxide
. These ligands are replaced by the alkyne in two steps forming first B and then C which enters the catalytic cycle
by oxidative coupling to the 16 VE cobaltacyclopentadiene metallacycle D. This compound forms in its singlet state
and therefore first relaxes to the triplet state
. This intermediate coordinates to another equivalent of ligand to form E and then forms cobaltanorbornene F (see: norbornadiene
) on accepting an alkyne unit in one [4+2]cycloaddition step. In the final step benzene
is liberated in a reductive elimination with formation of CpCoL which can re-enter the cycle by accepting two units of alkyne. When the ligand is less of a sigma donor such as ethylene
or THF
(as a solvent) or when the alkyne is electron-poor as in butynedioic acid a different cycle takes over where another alkyne unit and not a ligand adds to intermediate D. In the remainder of this cycle (not depicted) the new intermediate forms an CpCo(η4arene) complex and then a CpCo(η6arene) sandwich compound
before eliminating benzene and CpCo.
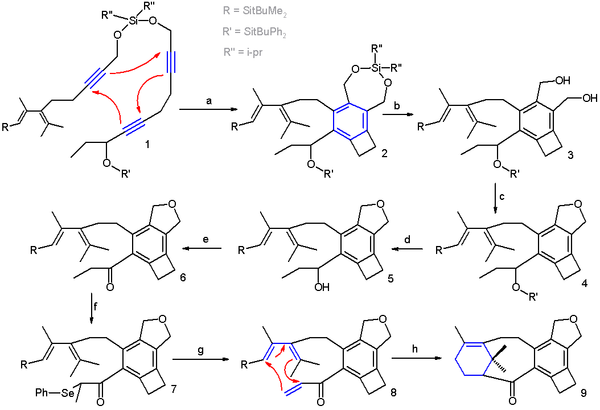
gives access to a Taxol
analogue (Scheme 2 ). In this reaction sequence the trimerization takes place in xylene
with catalyst the cobaltocene
CpCo(CO)2 (one Cp unit replaced by two carbonmonoxide ligand
s) and with irradiation. The two main components are held together by a temporary silicon tether.
 Dicobalt octacarbonyl
Dicobalt octacarbonyl
catalyzes the trimerization towards a hexakis(4-ferrocenylphenyl)benzene :

Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
of organometallic compounds containing a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
to cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12
Vitamin B12
Vitamin B12, vitamin B12 or vitamin B-12, also called cobalamin, is a water-soluble vitamin with a key role in the normal functioning of the brain and nervous system, and for the formation of blood. It is one of the eight B vitamins...
has a cobalt-carbon bond. Many organocobalt compounds are investigated for catalytic properties. An early example of organocobalt chemistry is the carbonylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
of azobenzene
Azobenzene
Azobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the best known example of an azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of molecules that share the core azobenzene structure, with different chemical...
with dicobalt octacarbonyl
Dicobalt octacarbonyl
Dicobalt octacarbonyl is the inorganic compound Co28. This metal carbonyl is a reagent and catalyst in organometallic chemistry and organic synthesis. It is used as a catalyst for hydroformylation, the conversion of alkenes to aldehydes....
as described by Murahashi & Horiie in 1956:

The basic characteristics of organocobalt compounds are:
- ease of forming complexes with alkynes, for example in the compound dicobalt hexacarbonyl diphenylacetylene , ease of forming complexes with dieneDieneIn organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
compounds and with cyclobutadieneCyclobutadieneCyclobutadiene is the smallest [n]-annulene , an extremely unstable hydrocarbon having a lifetime shorter than five seconds in the free state. It has chemical formula 44 and a rectangular structure verified by infrared studies. This is in contrast to the square geometry predicted by simple Hückel...
compounds, ease formation of sandwich compounds - high affinity of cobalt for carbonyls. Many cobalt metal carbonylMetal carbonylMetal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
s exist. - ease of formation of an octahedral molecular geometryOctahedral molecular geometryIn chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
with nitrogenNitrogenNitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
or oxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
at the base and Co-C bond formation in the axial position as in vitamin B12Vitamin B12Vitamin B12, vitamin B12 or vitamin B-12, also called cobalamin, is a water-soluble vitamin with a key role in the normal functioning of the brain and nervous system, and for the formation of blood. It is one of the eight B vitamins...
.
Triple bond complexes
Cobalt forms complexes with triple bondTriple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
ed alkynes and cyano compounds. This property is exploited in the use of dicobalt octacarbonyl
Dicobalt octacarbonyl
Dicobalt octacarbonyl is the inorganic compound Co28. This metal carbonyl is a reagent and catalyst in organometallic chemistry and organic synthesis. It is used as a catalyst for hydroformylation, the conversion of alkenes to aldehydes....
as protective group for alkynes. In the Nicholas reaction
Nicholas reaction
The Nicholas reaction is an organic reaction where a dicobalt octacarbonyl-stabilized propargylic cation is reacted with a nucleophile. Oxidative demetallation gives the desired alkylated alkyne.Several reviews have been published.-Reaction mechanism:...
an alkyne group is also protected and at the same time the alpha-carbon position is activated for nucleophilic substitution.
Cyclization reactions
Cobalt compounds react with dialkynes and dienes to cyclic intermediates in cyclometalation. Other alkynes, alkens, nitriles or carbon monoxide can then insert themselves into the Co-C bond. Reaction types based on this concept are the Pauson–Khand reactionPauson–Khand reaction
The Pauson–Khand reaction is a chemical reaction described as a [2+2+1] cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone...
(CO insertion) and alkyne trimerization (notably with cyclopentadienylcobalt dicarbonyl
Cyclopentadienylcobalt dicarbonyl
Cyclopentadienylcobalt dicarbonyl is an organocobalt compound with formula Co2, abbreviated CpCo2. It is an example of a half-sandwich complex. It is a dark red air sensitive liquid. This compound features one cyclopentadienyl ring that is bound in an η5-manner and two carbonyl ligands...
).
Carbonylations
Organocobalt compounds are used as catalysts in carbonylationCarbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
reactions and more specifically in hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
, the formation of aldehydes from an alkene, formaldehyde and hydrogen. An important catalyst in this reaction type is HCo(CO)4 (cobalthydrocarbonyl) at one time used in the industrial production of butyraldehyde from propylene
Propylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
. In these processes cobalt catalysts are competing with rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
catalysts such as HRh(CO)(PPh3)4].
In hydrocarboxylations hydrogen is replaced by water or an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
and the reaction product is a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
or an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
. An example of this reaction type is the conversion of butadiene to adipic acid
Adipic acid
Adipic acid is the organic compound with the formula 42. From the industrial perspective, it is the most important dicarboxylic acid: About 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon...
.
Cobalt catalysts (together with iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
) are relevant in the Fischer-Tropsch process
Fischer-Tropsch process
The Fischer–Tropsch process is a set of chemical reactions that convert a mixture of carbon monoxide and hydrogen into liquid hydrocarbons. The process, a key component of gas to liquids technology, produces a petroleum substitute, typically from coal, natural gas, or biomass for use as synthetic...
in which synthesis gas is converted to hydrocarbons. The basic reaction sequence is depicted below :
- (M = Co, Fe)
Vitamin B12-type compounds
In vitamin B12Vitamin B12
Vitamin B12, vitamin B12 or vitamin B-12, also called cobalamin, is a water-soluble vitamin with a key role in the normal functioning of the brain and nervous system, and for the formation of blood. It is one of the eight B vitamins...
cobalt has an octahedral geometry with a Co-C bond in an axial position. In methylcobalamin the ligand is a methyl group.
Sandwich compounds
Organocobalt compounds form sandwich compoundSandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives...
s. Cobaltocene
Cobaltocene
Cobaltocene, known also as biscobalt or even "bis Cp cobalt", is an organocobalt compound with the formula Co2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene...
is a 19-electron metallocene, the compound CoCp(C6(Me)6) ha 20 electrons and 21 electrons are counted in Co(C6(Me)6)2 . The Kläui ligand binds metals.
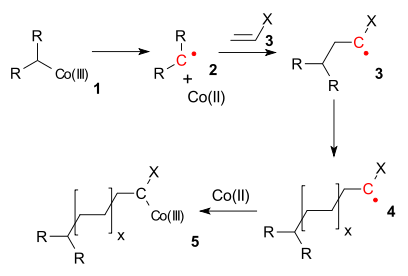
Cobalt-Mediated Radical Polymerization
The weak cobalt(III)- carbon bond is exploited in so-called cobalt-mediated radical polymerization (CMRP) which is a type of controlled radical polymerization. A Co-C bond containing radical initiatorRadical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
breaks up (by heat or by light) in a carbon free radical and a cobalt(II) radical species. The carbon radical starts polymer chain formation with monomer for instance an alkene as in any ordinary radical polymerization. Cobalt is unusual in that it can reversibly
Reversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
reform a covalent bond with the carbon radical terminus of the growing chain. This reduces the concentration of radicals to a minimum and also undesirable termination reactions by recombination of two carbon radicals. The cobalt trapping reagent is called a persistent radical and the cobalt-capped polymer chain is said to dormant. CMRP can be regarded as a series of carbometalation
Carbometalation
Carbometalation is an organometallic reaction involving the nucleophilic addition to alkenes and alkynes of a diverse range of organometallic reagents such as organolithium compounds, organocopper compounds and Grignard reagents according to the following general alkyne scheme:The addition can...
reactions of vinyl monomers. When the monomer possesses protons that can be easily abstracted by the cobalt radical, (catalytic) chain transfer
Chain transfer
Chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule.Chain transfer reactions reduce the average molecular weight of the final polymer...
may occur.
For this reason preferred monomers are acrylic and vinyl esters (e.g. vinyl acetate
Vinyl acetate
Vinyl acetate is an organic compound with the formula CH3COOCH=CH2. A colorless liquid with a pungent odor, it is the precursor to polyvinyl acetate, an important polymer in industry.-Production:...
), acrylic acid
Acrylic acid
Acrylic acid is an organic compound with the formula CH2=CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols,...
and acrylonitrile
Acrylonitrile
Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile...
. The reaction temperature is typically between 0 and 60 °C.
The concept was introduced independently by two groups in 1994.
Much studied cobalt compounds are cobaloximes, cobalt porphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
s and Co(acac
Acac
ACAC may refer to:* American Council for Accredited Certification, a private, non-profit certifying body* Atlanta Contemporary Art Center, a contemporary art museum in Atlanta* ACAC consortium, a subsidiary of China Aviation Industry Corporation...
)2 derivatives, used in combination with other radical initiators.
Cobalt mediated radical polymerization can also follow different mechanisms, such as catalytic chain transfer
Catalytic Chain Transfer
Catalytic chain transfer is a process that can be incorporated into radical polymerization to obtain greater control over the resulting products.-Introduction:...
or degenerative transfer:
Cyclotrimerisation
Cobalt compounds are catalysts in alkyne trimerisationAlkyne trimerisation
An alkyne trimerisation reaction is a 2+2+2 cyclization reaction in which three alkyne molecules react to form an aromatic compound. The reaction is 'pseudo' pericyclic since it has not been observed to occur without the assistance of metal catalysis; and the metal catalyst assembles the ring...
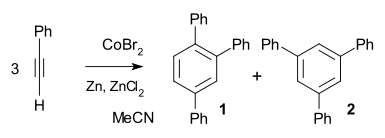
. In the example of phenylacetylene
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.-Preparation:...
a simple catalyst system of cobalt(II) bromide
Cobalt(II) bromide
Cobalt bromide is an inorganic compound used primarily as a catalyst in some processes.-Properties:When anhydrous, cobalt bromide appears as green crystals. The hexahydrate loses four waters of crystallization molecules at 100°C forming the dihydrate:Further heating to 130°C Produces the anhydrous...
/ zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
/ zinc iodide
Zinc iodide
Zinc iodide is a chemical compound of zinc and iodine, ZnI2. The anhydrous form is white and readily absorbs water from the atmosphere. It can be prepared by the direct reaction of zinc and iodine in refluxing ether...
suffices to obtain 99% chemical yield and 97% regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
in favor of the ortho substituted reaction product 1.

Mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for trimerization is fairly well understood. A 2007 mechanism consistent with in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
and experimental data is depicted below for a cobaltocene
Cobaltocene
Cobaltocene, known also as biscobalt or even "bis Cp cobalt", is an organocobalt compound with the formula Co2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene...
catalyst

Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
L in the 18-electron compound
Electron counting
Electron counting is a formalism used for classifying compounds and for explaining or predicting electronic structure and bonding. Many rules in chemistry rely on electron-counting:...
CpCoL2 (A) can be triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
or carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
. These ligands are replaced by the alkyne in two steps forming first B and then C which enters the catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
by oxidative coupling to the 16 VE cobaltacyclopentadiene metallacycle D. This compound forms in its singlet state
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
and therefore first relaxes to the triplet state
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
. This intermediate coordinates to another equivalent of ligand to form E and then forms cobaltanorbornene F (see: norbornadiene
Norbornadiene
Norbornadiene is an organic compound. This bicyclic hydrocarbon is the most stable diolefin derived from the norbornane and norbornene. Norbornadiene is primarily of interest as a ligand in homogeneous catalysis, but it has been heavily studied due to its high reactivity and distinctive...
) on accepting an alkyne unit in one [4+2]cycloaddition step. In the final step benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
is liberated in a reductive elimination with formation of CpCoL which can re-enter the cycle by accepting two units of alkyne. When the ligand is less of a sigma donor such as ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
or THF
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
(as a solvent) or when the alkyne is electron-poor as in butynedioic acid a different cycle takes over where another alkyne unit and not a ligand adds to intermediate D. In the remainder of this cycle (not depicted) the new intermediate forms an CpCo(η4arene) complex and then a CpCo(η6arene) sandwich compound
Sandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives...
before eliminating benzene and CpCo.
Scope
In one study, a combination of a [2+2+2] trimerization and a [4+2] cycloadditionDiels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
gives access to a Taxol
Taxol total synthesis
Paclitaxel total synthesis in organic chemistry is a major ongoing research effort in the total synthesis of paclitaxel . This diterpenoid is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the Pacific yew...
analogue (Scheme 2 ). In this reaction sequence the trimerization takes place in xylene
Xylene
Xylene encompasses three isomers of dimethylbenzene. The isomers are distinguished by the designations ortho- , meta- , and para- , which specify to which carbon atoms the two methyl groups are attached...
with catalyst the cobaltocene
Cobaltocene
Cobaltocene, known also as biscobalt or even "bis Cp cobalt", is an organocobalt compound with the formula Co2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene...
CpCo(CO)2 (one Cp unit replaced by two carbonmonoxide ligand
Metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
s) and with irradiation. The two main components are held together by a temporary silicon tether.

Dicobalt octacarbonyl
Dicobalt octacarbonyl is the inorganic compound Co28. This metal carbonyl is a reagent and catalyst in organometallic chemistry and organic synthesis. It is used as a catalyst for hydroformylation, the conversion of alkenes to aldehydes....
catalyzes the trimerization towards a hexakis(4-ferrocenylphenyl)benzene :

See also
- Chemical bonds of carbon with other elements in the periodic table: